- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Vardenafil HCl Trihydrate PDE inhibitor
Cat.No.S2515
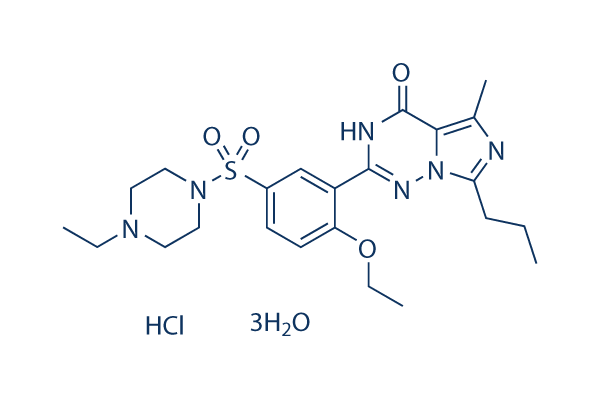
Chemical Structure
Molecular Weight: 579.11
Jump to
Quality Control
Batch:
Purity:
99.66%
99.66
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(172.67 mM)
Ethanol : 100 mg/mL Water : 10 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 579.11 | Formula | C23H32N6O4S.HCl.3H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 330808-88-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BAY38-9456 | Smiles | CCCC1=NC(=C2N1N=C(NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)CC)OCC)C.O.O.O.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
PDE5
(Cell-free assay) 0.7 nM
|
|---|---|
| In vitro |
Vardenafil specifically inhibits the hydrolysis of cGMP by PDE5 with an IC50 of 0.7 nM (6.6 nM). Vardenafil significantly enhances the SNP-induced relaxation of human trabecular smooth muscle at 3 nM (10 nM). Vardenafil also significantly potentiates both ACh-induced and transmural electrical stimulation-induced relaxation of trabecular smooth muscle. Vardenafil (100 mM) increases cyclic GMP levels in rat hippocampal slices. Vardenafil, tadalafil, and Sildenafil each competitively inhibit cGMP hydrolysis by phosphodiesterase-5 (PDE5), thereby fostering cGMP accumulation and relaxation of vascular smooth muscle.
|
| In vivo |
Vardenafil dose-dependently potentiates erectile responses to intravenously administered sodium nitroprusside in rabbit. Vardenafil (3 mg/kg, p.o.) results in an improved object discrimination performance in rats. Vardenafil (30 mg/L, p.o.) increases both iNOS and proliferating cell nuclear antigen expression (SM cell replication) in rats, with normalization of the dynamic infusion cavernosometry drop rate and SM/collagen ratio. Vardenafil induces powerful preconditioning-like cardioprotective effect against ischemia/reperfusion injury through opening of mitochondrial K(ATP) channels in the heart of rabbit. Vardenafil protects the ischemic myocardium against reperfusion injury through a mechanism dependent on mitochondrial K(ATP) channel opening.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02279992 | Terminated | Glioma|Brain Neoplasms|Brain Metastasis |
Cedars-Sinai Medical Center |
March 27 2012 | Early Phase 1 |
| NCT01348880 | Completed | Erectile Dysfunction |
Bayer|GlaxoSmithKline|Merck Sharp & Dohme LLC |
May 2011 | Phase 1 |
| NCT01106118 | Completed | Erectile Dysfunction |
Bayer |
January 2010 | -- |
| NCT01084187 | Completed | Erectile Dysfunction|Arterial Hypertension|Endothelial Dysfunction |
Hospital Universitario Pedro Ernesto |
January 2010 | Phase 4 |
| NCT00738400 | Completed | Erectile Dysfunction|Metabolic Syndrome |
Bayer |
November 2008 | Phase 4 |
| NCT00718952 | Completed | Pulmonary Hypertension |
Tongji University |
July 2008 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































