- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Ibudilast PDE inhibitor
Cat.No.S4837
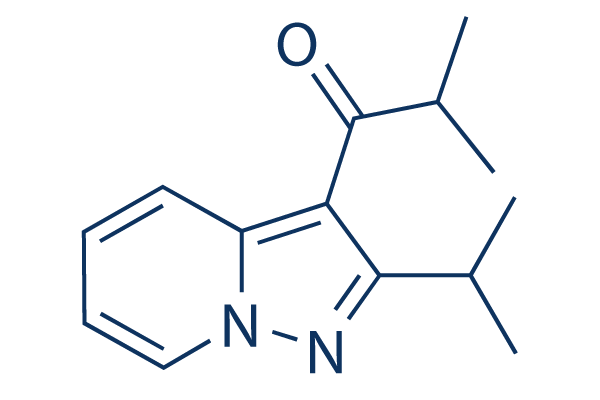
Chemical Structure
Molecular Weight: 230.31
Jump to
Quality Control
Batch:
Purity:
99.91%
99.91
Solubility
|
In vitro |
DMSO
: 46 mg/mL
(199.73 mM)
Ethanol : 46 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 230.31 | Formula | C14H18N2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 50847-11-5 | -- | Storage of Stock Solutions |
|
|
| Synonyms | KC-404, AV411, MN166 | Smiles | CC(C)C1=NN2C=CC=CC2=C1C(=O)C(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
PDE4
(Bovine aortic EC) 0.08 μM
PDE2
(Bovine aortic EC) 0.11 μM
PDE5
(Human platelets) 2.2 μM
|
|---|---|
| In vitro |
Ibudilast suppressses pro-inflammatory cytokines (IL-6, IL-1β, TNF-α), toll-like receptor 4 blockade (TLR-4); inhibits a macrophage migration inhibitory factor (MIF), upregulates the anti‑inflammatory cytokine (IL-10) and promotes neurotrophic factors (GDNF, NGF, NT‑4).
|
| In vivo |
Ibudilast crosses the blood-brain barrier and has a good tolerance in vivo. It inhibits platelet aggregation, improves cerebral blood flow and attenuates allergic reactions in clinical applications. In an animal model of encephalomyelitis, this compound significantly attenuates inflammatory cell infiltration in the lumbar spinal cord. After oral administration, it is well absorbed and metabolized in the liver mainly to diOH-ibudilast, which has similar or even stronger pharmacological effects.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04054206 | Completed | Healthy Volunteers |
MediciNova |
June 24 2019 | Phase 1 |
| NCT03341078 | Recruiting | Methamphetamine-dependence |
VA Office of Research and Development|Portland VA Medical Center|Oregon Health and Science University |
May 1 2019 | Phase 2 |
| NCT03782415 | Active not recruiting | Glioblastoma|Recurrent Glioblastoma|GBM|Newly Diagnosed Glioblastoma |
MediciNova |
December 29 2018 | Phase 1|Phase 2 |
| NCT02714036 | Completed | Amyotrophic Lateral Sclerosis |
MediciNova|Massachusetts General Hospital|South Shore Neurologic Associates |
May 6 2016 | Phase 1|Phase 2 |
| NCT00723177 | Completed | Opioid-Related Disorders |
New York State Psychiatric Institute|National Institute on Drug Abuse (NIDA) |
October 2008 | Phase 2 |
| NCT00576277 | Completed | Diabetic Neuropathy |
Avigen |
September 2006 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































