research use only
Anti-DYKDDDDK Tag magnetic beads
Anti-DYKDDDDK Tag magnetic beads is based on hydroxyl magnetic beads covalently coupling with high quality recombinant mouse monoclonal antibody. With high loading of DYKDDDDK-tagged protein (more than 0.6 mg protein/mL) and high specificity, it is recommended to use for co-immunoprecipitation and protein purification.
Selleck's Has Been Cited by 119 Publications
Advantages
Time saving: save 15-30 min comparing with agarose beads.
Simple operation: Magnetic separation and centrifugation-free.
High protein loading capacity.
High specificity.
Price Comparison
Description
Anti-DYKDDDDK Tag magnetic beads is based on hydroxyl magnetic beads covalently coupling with high quality recombinant mouse monoclonal antibody. With high loading of DYKDDDDK-tagged protein (more than 0.6 mg protein/mL) and high specificity, it is recommended to use for co-immunoprecipitation and protein purification.
Properties
| Antibody isotype | Recombinant mouse monoclonal antibody |
|---|---|
| Antibody purification | Protein A purified |
| Application | Immunoprecipitation and protein Purification |
| Recommended volume | IP: 20 μl beads for 200 μl crude protein solution |
| Binding capacity | Minimum 0.6 mg protein eluted per ml of magnetic beads |
| Binding characteristics | Met-N-terminal DYKDDDDK Tag fusion protein: Met-DYKDDDDK Tag–Protein N-terminal DYKDDDDK Tag fusion protein: DYKDDDDK Tag–Protein C-terminal DYKDDDDK Tag fusion protein: Protein-DYKDDDDK Tag |
Storage (From the date of receipt)
Store at 2-8°C for 2 years. DO NOT freeze or centrifuge Magnetic Beads.
Protocol
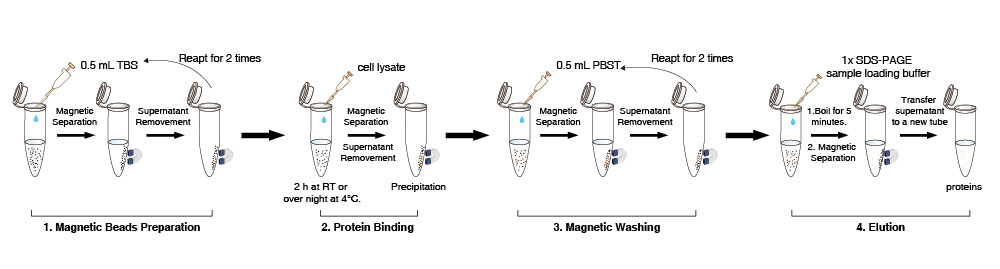
Magnetic Beads Preparation
1. Suspend the Anti-DYKDDDDK Tag magnetic beads in the vial (pipet gently for 10 times, don’t vortex). Transfer 10 µL (the amount may be scaled up or down as required) Anti-DYKDDDDK Tag Magnetic Beads suspension to a new tube.
2. Add 0.5 mL TBS buffer (50 mM Tris HCl, 150 mM NaCl, pH 7.4). Pipet gently for 5 times Anti-DYKDDDDK Tag magnetic beads. Place the tube on the magnet to separate the beads from the solution for 1-2 min (can be appropriately extended to 5 minutes) and remove the supernatant. Repeat this step for 2 times.
Note: Prepare all Magnetic Beads together in one large tube and then divide it into aliquots if samples are in batch. When removing the supernatant, please suck gently, as excessive suction may result in the loss of some magnetic beads.
Protein Binding
3. Add 500 µL of cell lysate to the washed magnetic beads. Gently rotate the tube for 2 h at room temperature or over night at 4°C.
4. Place the tube on the magnet to separate the beads from the solution for 1-2 min (can be appropriately extended) and then transfer the supernatant into a new tube for detecting whether DYKDDDDK-tag protein is residual.
Note:During the binding process, it won't affect the result if magnetic beads occasionally cluster together.
Magnetic Washing
5. Add 500 µL PBST to the tube (NaCl 136.89 mM; KCl 2.67 mM; Na2HPO4 8.1 mM; KH2PO4 1.76 mM;0.5% Tween20), resuspend the magnetic beads by pipeting gently. Then rotate the tube for 5 min. Place the tube on the magnet to separate the beads from solution for 10 sec and remove the supernatant.
6. Repeat step 5 for about 2 times. If the non-specific impurity proteins are left over, please extend the cleaning time, increase the cleaning times or properly enlarge the detergent content in the cleaning solution.
Elution and Detectione
Choose different elution methods according to the downstream use. For IP, go to the step 7- 8. For protein purification, go to step 9-10 for low pH elution.
Denaturing elution (suitable for IP experiments using Anti-DYKDDDDK Tag beads):
7. For direct detection of target proteins, add 50 µL 1×protein sample loading buffer in the precipitation mentioned above, boil for 5 min, chill to room temperature and then place the tube on the magnet to separate the beads from the solution for 1-2 min (can be appropriately extended).
8. Detect the supernatant by SDS-PAGE.
Competitive poly DYKDDDDK Tag polypeptide elution (Suitable for protein purifications by Anti-DYKDDDDK Tag beads):
9. Add the TBS buffer with 200 µg-1 mg/mL Poly DYKDDDDK Tag Peptide (B23111) into the product of step 6, and then incubate them at shaker (4°C) for 2 h. Generally, the volume of Poly DYKDDDDK Tag Peptide is 5 times of the gel.
10. Place products of the above step on the magnet separation for magnetic separation. Transfer the supernatant containing target protein into a new EP tube. If needing reuse the magnetic beads, please clean the gel with 0.1 M glycine HCl (pH 3.0) and carry out recycling.
Low-pH elution (Suitable for protein purification by Anti-DYKDDDDK Tag beads):
11. Add the 0.1 M glycine HCl (pH 3.0) elution buffer into the product of step 6, and incubate at shaker for 5 min (The elution time should be less than 20 min). Generally, the volume of elution buffer is 5 times of the gel.
12. Centrifugation the products obtained from the above step at 5000 rpm for 30 sec. Then transfer the elution product quickly into 1 M Tris (pH 8.0) for neutralization until the pH is near neutral.
Trouble Shooting
| Problem | Possible Reason | Suggested Improvement |
|---|---|---|
| High background | Nonspecifically binding of proteins to the antibody, megnetic beads or EP tubes | Pre-clear lysate to remove nonspecific binding proteins. After suspending beads for the final wash, transfer the entire sample to a clean EP tube and then magnetic separation. |
| Washing times are not sufficient. | Increase the number of washes. Increase duration time of washes. |
|
| No signal is observed. | DYKDDDDK-tagged protein is not expressed in the sample. | Make sure the protein of interest contains the DYKDDDDK Tag sequence. Prepare the fresh lysate. Use appropriate protease inhibitors. |
| Incubation times are inadequate. | Increase the incubation times. | |
| Interfering substance is present in sample. | The lysate may contain high concentrations of dithiothreitol (DTT), 2-mercaptoethanol, or other reducing agents. Excessive detergent concentration may interfere with the antibody-antigen interaction. |
File Download
Related Other Products
Tech Support
If you have any other enquiries, please leave a message.





































