research use only
VPA (Valproic acid) HDAC inhibitor
Cat.No.S3944
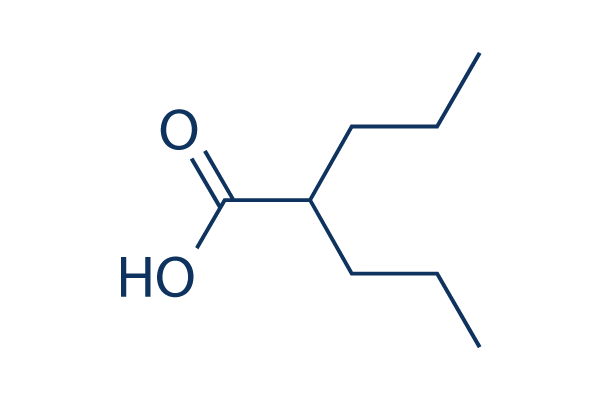
Chemical Structure
Molecular Weight: 144.21
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 | Function assay | 1 mM | Increase in protein disulfide isomerase level in HEK293 cells at 1 mM by immunoblot | 17566732 | ||
| HEK293 | Function assay | 1 mM | Increase in GRP78 protein level in HEK293 cells at 1 mM by immunoblot | 17566732 | ||
| A549 | Function assay | 150 uM | 24 hrs | Inhibition of human HDAC in A549 cells assessed as increase in histone-H4 acetylation at 150 uM after 24 hrs by Western blot | 18294844 | |
| GM15850 | Function assay | 400 uM | 12 hrs | Inhibition of HDAC in human GM15850 cells assessed as increase in total acetylated histone level at 400 uM after 12 hrs by Western blot analysis | 16921367 | |
| PC12 | Function assay | 1 uM | 24 hrs | Induction of autophagy in rat stable inducible PC12 cells expressing A53T alpha-synuclein assessed as A53T alpha-synuclein clearance at 1 uM after 24 hrs by densitometric analysis | 18391949 | |
| PC12 | Function assay | 1 uM | 96 hrs | Induction of autophagy in rat stable inducible PC12 cells expressing EGFP-HDQ74 assessed as soluble EGFP-HDQ74 clearance at 1 uM after 96 hrs by densitometric analysis | 18391949 | |
| SK-N-MC | Function assay | 1 mM | 48 hrs | Induction of autophagy in human SK-N-MC cells expressing EGFP-HDQ74 assessed as reduction in EGFP-HDQ74 aggregation at 1 uM after 48 hrs by densitometric analysis | 18391949 | |
| HL60 | Function assay | 1 mM | 24 hrs | Inhibition of HDAC in human HL60 cells assessed as increase in histone H3 acetylation at 1 mM after 24 hrs by Western blotting method | 25304896 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 29 mg/mL
(201.09 mM)
Water : 29 mg/mL Ethanol : 29 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 144.21 | Formula | C8H16O2 |
Storage (From the date of receipt) | 2 years -20°C liquid |
|---|---|---|---|---|---|
| CAS No. | 99-66-1 | -- | Storage of Stock Solutions |
|
|
| Synonyms | 2-Propylvaleric Acid, Valproate | Smiles | CCCC(CCC)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
HDAC
HDAC1
(Cell-free assay) 0.4 mM
|
|---|---|
| In vitro |
Like lithium, valproic acid (VPA) activates Wnt-dependent gene expression, but unlike lithium, it does not inhibit GSK-3β in vivo. This compound can inhibit GSK-3β-mediated phosphorylation of a CREB peptide in vitro. It may activate Wnt-dependent gene expression through inhibition of HDAC, which in turn leads to both increased expression of β-catenin and de-repression of Tcf/Lef (as well as activation of other HDAC-regulated genes). In vitro, VPA can stimulate glutamic acid decarboxylase, which is involved in GABA biosynthesis, and inhibit GABA transaminase, succinic semialdehyde dehydrogenase, and α-ketoglutarate dehydrogenase, enzymes involved in GABA degradation. It relieves HDAC-dependent transcriptional repression and causes hyperacetylation of histones in cultured cells and in vivo. VPA induces differentiation and/or apoptosis of carcinoma cells, PML-RAR-transformed hematopoietic progenitor cells and leukemic blasts from AML patients. In addition to selectively inhibiting the catalytic activity of class I HDACs, it also induces proteasomal degradation of HDAC2.
|
| In vivo |
Valproic acid (VPA) increases the level of the inhibitory neurotransmitter γ-aminobutyric acid (GABA), with acute administration causing a 15-45% increase in GABA in the brains of rodents. It also inhibits tumor growth and metastasis in animal experiments, and is a well-tolerated drug even during long-term treatment.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | acetyl-H4 Acetyl-H3 p-IKKα/β / IKKα/β / NF-κB p65 / IκBα |
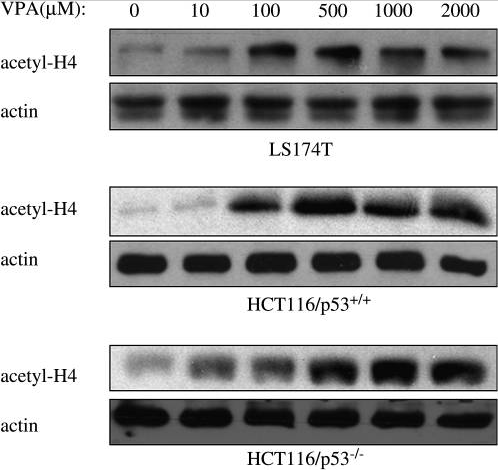
|
20025549 |
| Growth inhibition assay | Cell viability |
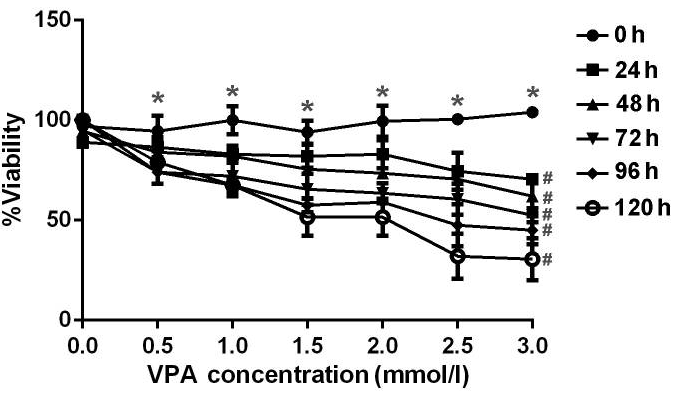
|
28101176 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04671589 | Unknown status | Drug Toxicity |
Mabaret Al-Asafara Hospitals |
June 2021 | Phase 4 |
| NCT04384172 | Recruiting | Female Sexual Dysfunction|Spinal Cord Injuries |
University of Michigan|International Society for the Study of Women''s Sexual Health|The Craig H. Neilsen Foundation |
November 11 2020 | Not Applicable |
| NCT03962829 | Terminated | Eating Behavior|Obesity |
University of Birmingham|University Hospital Birmingham |
February 1 2019 | Not Applicable |
| NCT03681158 | Completed | Epilepsy |
Sanofi |
October 5 2018 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































