research use only
Pracinostat (SB939) HDAC inhibitor
Cat.No.S1515
Chemical Structure
Molecular Weight: 358.48
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MCF7 cells | Proliferation assay | 48 h | Antiproliferative activity against human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50=0.17 μM | 24119555 | ||
| NCI-H460 cells | Proliferation assay | 48 h | Antiproliferative activity against human NCI-H460 cells after 48 hrs by sulforhodamine B assay, GI50=0.22 μM | 24119555 | ||
| HCT116 cells | Proliferation assay | 48 h | Antiproliferative activity against human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50=0.24 μM | 24119555 | ||
| MDA-MB-435 cells | Proliferation assay | 48 h | Antiproliferative activity against human MDA-MB-435 cells after 48 hrs by sulforhodamine B assay, GI50=0.48 μM | 24119555 | ||
| OVCAR5 cells | Proliferation assay | 48 h | Antiproliferative activity against human OVCAR5 cells after 48 hrs by sulforhodamine B assay, GI50=0.61 μM | 24119555 | ||
| HepG2 | Antimalarial assay | Antimalarial activity against exo-erythrocytic form of Plasmodium berghei infected in human HepG2 cells, IC50 = 0.15 μM. | 24904967 | |||
| HepG2 | Antiplasmodial assay | 53 hrs | Antiplasmodial activity against exoerythrocytic-stage of Plasmodium berghei ANKA infected in human HepG2 cells after 53 hrs by DAPI staining-based method, IC50 = 0.15 μM. | 28241112 | ||
| Sf9 | Function assay | 2 hrs | Inhibition of recombinant human HDAC-2 expressed in baculovirus infected insect Sf9 cells using Fluor de Lys as substrate preincubated for 2 hrs followed by substrate addition measured after 10 mins by fluorescence assay, Ki = 0.016 μM. | ChEMBL | ||
| HEK293-F | Function assay | 2 hrs | Inhibition of recombinant human HDAC-8 expressed in HEK293-F cells using Fluor de Lys as substrate preincubated for 2 hrs followed by substrate addition measured after 10 mins by fluorescence assay, Ki = 0.016 μM. | ChEMBL | ||
| Sf9 | Function assay | 2 hrs | Inhibition of recombinant human HDAC-1 expressed in baculovirus infected insect Sf9 cells using Fluor de Lys as substrate preincubated for 2 hrs followed by substrate addition measured after 10 mins by fluorescence assay, Ki = 0.016 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 72 mg/mL
(200.84 mM)
Ethanol : 27 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 358.48 | Formula | C20H30N4O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 929016-96-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCCCC1=NC2=C(N1CCN(CC)CC)C=CC(=C2)C=CC(=O)NO | ||
Mechanism of Action
| Features |
A new histone deacetylase inhibitor based on hydroxamic acid, with improved physicochemical, pharmaceutical, and pharmacokinetic properties.
|
|---|---|
| Targets/IC50/Ki |
HDAC10
(Cell-free assay) 40 nM
HDAC3
(Cell-free assay) 43 nM
HDAC5
(Cell-free assay) 47 nM
HDAC1
(Cell-free assay) 49 nM
HDAC4
(Cell-free assay) 56 nM
HDAC9
(Cell-free assay) 70 nM
HDAC11
(Cell-free assay) 93 nM
HDAC2
(Cell-free assay) 96 nM
HDAC7
(Cell-free assay) 137 nM
HDAC8
(Cell-free assay) 140 nM
HDAC6
(Cell-free assay) 1.008 μM
|
| In vitro |
Pracinostat (SB939) has a 100-fold greater selectivity for HDACs than for Zn-binding non-HDAC enzymes, receptors, and ion channels. This compound is a potent inhibitor of HDAC class I isoenzymes, HDAC1, HDAC2, HDAC3 and HDAC8 with the IC50 values ranging from 43 nM to 140 nM. It inhibits HDAC class II isoenzymes , HDAC4, HDAC5, HDAC7, HDAC9 and HDAC10 significantly with the IC50 values ranging from 40 nM to 137 nM, with the exception of HDAC6 which shows IC50 of 1008 nM. It markedly inhibits HDAC11 of the HDAC class IV enzymes with IC50 of 93 nM, but shows no inhibitory activity against SIRT 1 of the class III HDACs. This chemical shows significant antiproliferative activity against a wide variety of tumor cell lines, especially Leukemia cells and cutaneous T-cell Lymphoma cells with IC50 values ranging from 50 nM (H9 cells) to 170 nM (HEL92.1.7 cells). |
| Kinase Assay |
HDAC enzyme assay
|
|
All recombinant HDAC enzymes, with the exception of SIRT1, are cloned and expressed in S*BIO. The reaction mix containing 2.5 or 5 μL of the HDAC isoenzyme, assay buffer (25 mM Tris-HCl, pH 7.5; 137 mM NaCl; 2.7 mM KCl, 1 mM MgCl2 and 1 mg/mL BSA), different concentrations of this compound, and the fluorogenic deacetylase substrate Flour de LysTM in a total reaction volume of 33 μL is incubated at room temperature for 2 hours. 16 μL of Flour de LysTM developer is added and incubated for an additional 10 minutes. The emitted light is measured at 460 nm in a microplate reader. IC50 values are generated using the XLfit software.
|
|
| In vivo |
Administration of this compound (25 mg/kg to 100 mg/kg) displays a dose-dependent antitumor efficacy in a xenograft mice model of human colorectal cancer (HCT-116). This is approximately twice as efficacious as SAHA: this chemical causing a tumor growth inhibition of 94% versus 48% by SAHA with both at the maximum tolerated dose. Oral administration of this agent at a dose of 50 mg/kg or 75 mg/kg in the APCmin genetic mice model of early-stage colon cancer markedly reduces the number of tumors , decreases cumulative hemocult scores and increases hematocrit values more effectively. |
References |
Applications
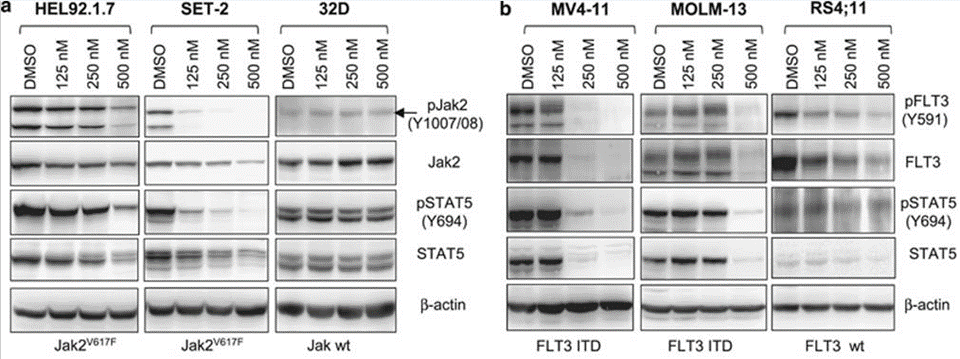
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-JAK2 / JAK2 / p-STAT5 / STAT5 / p-FLT3 / FLT3 |
|
22829971 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02118909 | Completed | Healthy Volunteers|Non-smokers |
Helsinn Healthcare SA |
May 2014 | Phase 1 |
| NCT02058784 | Completed | Healthy Volunteers|Moderate to Heavy Smokers|Non-smokers |
Helsinn Healthcare SA|Celerion |
February 2014 | Early Phase 1 |
| NCT01112384 | Completed | Metastatic Sarcoma |
NCIC Clinical Trials Group|S*BIO|Canadian Cancer Trials Group |
October 21 2010 | Phase 2 |
| NCT00741234 | Completed | Solid Tumors|Hematologic Malignancies|Myelodysplastic Syndrome |
S*BIO |
April 2007 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































