research use only
Sulfasalazine Immunology & Inflammation related inhibitor
Cat.No.S1576
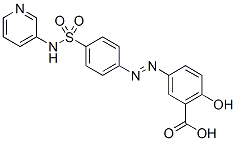
Chemical Structure
Molecular Weight: 398.39
Quality Control
| Related Targets | PD-1/PD-L1 CXCR STING AhR CD markers Interleukins Anti-infection Antioxidant COX Histamine Receptor |
|---|---|
| Other Immunology & Inflammation related Inhibitors | Cl-amidine Bestatin (Ubenimex) Bindarit (AF 2838) Tranilast Tempol Sinomenine GI254023X (GI4023) ATP Geniposidic acid CORM-3 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 Flp-In | Function assay | Inhibition of human liver OATP1B1 expressed in HEK293 Flp-In cells assessed as reduction in E17-betaG uptake by scintillation counting, Ki=0.53μM | 22541068 | |||
| HEK293 Flp-In | Function assay | Inhibition of human liver OATP1B1 expressed in HEK293 Flp-In cells assessed as reduction in E17-betaG uptake by scintillation counting, IC50=0.56μM | 22541068 | |||
| HepG2 | Function assay | 1 hr | Inhibition of NF-kappaB activation expressed in TNFalpha-stimulated human HepG2 cells co-transfected with PPRE-Luc plasmid pretreated for 1 hr before TNFalpha challenge measured after 24 hrs by luciferase reporter gene assay, IC50=0.9μM | 21889336 | ||
| HepG2 | Function assay | 1 hr | Inhibition of TNF-alpha-induced NFkappaB activation in human HepG2 cells after 1 hr by luciferase reporter assay, IC50=0.9μM | 21870831 | ||
| HepG2 | Function assay | 1 hr | Inhibition of TNFalpha-induced NFkappaB transcriptional activity in human HepG2 cells pretreated for 1 hr before TNFalpha challenge by luciferase reporter gene analysis, IC50=0.9μM | 22381047 | ||
| HepG2 | Function assay | 1 hr | Inhibition of NF-kappaB expressed in human HepG2 cells assessed as inhibition of TNFalpha-induced luciferase activity preincubated for 1 hr followed by TNFalpha challenge measured after 1 hr by reporter gene assay, IC50=0.9μM | 23031596 | ||
| HepG2 | Function assay | 1 hr | Inhibition of TNF-alpha-induced NF-kappaB transcriptional activation in human HepG2 cells preincubated for 1 hr followed by TNF-alpha challenge measured after 1 hr by luciferase reporter gene assay, IC50=0.9μM | 24314396 | ||
| HEK293 Flp-In | Function assay | 5 mins | Inhibition of human liver OATP2B1 expressed in HEK293 Flp-In cells assessed as reduction in [3H]E3S uptake incubated for 5 mins by scintillation counting, Ki=3μM | 22541068 | ||
| HEK293 Flp-In | Function assay | 5 mins | Inhibition of human liver OATP2B1 expressed in HEK293 Flp-In cells assessed as reduction in [3H]E3S uptake incubated for 5 mins by scintillation counting, IC50=3μM | 22541068 | ||
| U2OS | Function assay | 10 mins | Inhibition of HA-tagged human NTCP expressed in human U2OS cells assessed as reduction in [14C]taurocholate uptake preincubated for 10 mins followed by [14C] taurocholate addition and further incubation for 10 mins by scintillation counting method, IC50=9.6μM | 31439379 | ||
| HEK293 Flp-In | Function assay | 5 mins | Inhibition of human liver OATP1B3 expressed in HEK293 Flp-In cells assessed as reduction in [3H]E17-betaG uptake incubated for 5 mins by scintillation counting, Ki=11μM | 22541068 | ||
| HEK293 Flp-In | Function assay | 5 mins | Inhibition of human liver OATP1B3 expressed in HEK293 Flp-In cells assessed as reduction in [3H]E17-betaG uptake incubated for 5 mins by scintillation counting, IC50=12μM | 22541068 | ||
| U87 | Function assay | 15 mins | Inhibition of xc-cystine-glutamate antiporter-mediated cystine uptake in human U87 cells using L-[14C]cystine as substrate after 15 mins by liquid scintillation counting, IC50=30μM | 21889337 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 80 mg/mL
(200.8 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 398.39 | Formula | C18H14N4O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 599-79-1 | Download SDF Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 667219, Sulphasalazine | Smiles | C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N=NC3=CC(=C(C=C3)O)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
COX-2
TGF-β
|
|---|---|
| In vitro |
Sulfasalazine, like methotrexate, enhances adenosine release at an inflamed site and that adenosine diminishes inflammation via occupancy of A2 receptors on inflammatory cells. This compound treatment for 4 hours inhibits kappaB-dependent transcription with an IC50 value of approximately 0.625 mM. This chemical (2.5 mM) results in cell death of T-lymphocytes in a dose- and time-dependent manner. This compound but not 5ASA or sulfapyridine, strongly inhibits NF-kappaB activation and potently induces apoptosis in T-lymphocytes. It is cleaved into sulfapyridine and 5-aminosalicylic acid (5-ASA; mesalamine) by colonic bacteria, and the latter, too, is reported to suppress NF-kappaB activity. This compound but not its cleaved form 5-ASA causes a dose-dependent inhibition of glioma growth, this effect is entirely attributable to the inhibition of cystine uptake via the system x(c)(-) cystine-glutamate transporter. It inhibits cystine uptake causing a chronic depletion of intracellular GSH and consequently compromised cellular redox defense which stymied tumor growth. |
| In vivo |
Sulfasalazine markedly decreases the number of leukocytes that accumulated in the inflamed (carrageenan, 2 mg/ml) air pouch in the murine air pouch model of inflammation. This compound treatment promotes a marked increase in splenocyte 5-aminoimidazole-4-carboxamidoribonucleotide (AICAR) concentration, which is consistent with the in vitro observation that it inhibits AICAR transformylase. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06134388 | Recruiting | Metastatic Colorectal Cancer |
Tanta University |
September 1 2023 | Phase 3 |
| NCT03487926 | Active not recruiting | Major Depressive Episode|Inflammatory Bowel Diseases |
Centre for Addiction and Mental Health|McMaster University |
September 14 2022 | -- |
| NCT04720183 | Completed | Healthy |
Galapagos NV |
January 11 2021 | Phase 1 |
| NCT01577966 | Completed | Brain Tumor |
University of Alabama at Birmingham |
January 2012 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































