research use only
Bindarit (AF 2838) MCP/CCL Inhibitor
Cat.No.S3032
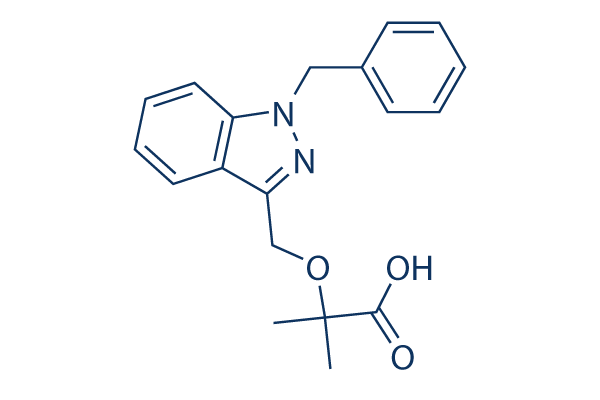
Chemical Structure
Molecular Weight: 324.37
Quality Control
| Related Targets | PD-1/PD-L1 CXCR STING AhR CD markers Interleukins Anti-infection Antioxidant COX Histamine Receptor |
|---|---|
| Other Immunology & Inflammation related Inhibitors | Cl-amidine Bestatin (Ubenimex) Tranilast Tempol Sinomenine GI254023X (GI4023) ATP Geniposidic acid CORM-3 Acacetin |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 64 mg/mL
(197.3 mM)
Ethanol : 64 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 324.37 | Formula | C19H20N2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 130641-38-2 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Features |
Bindarit is devoid of immunosuppressive effects.
|
|---|---|
| Targets/IC50/Ki |
MCP-1/CCL2
MCP-3/CCL7
MCP-2/CCL8
|
| In vitro |
Bindarit treatment causes a dose-dependent inhibition of the capacity of human monocytes to produce monocyte chemotactic protein-1 (MCP-1) in response to bacterial LPS or C. albicans with IC50 of 172 µM and 403 µM, respectively. The inhibition of LP-induced MCP-1 production by this compound is associated with reduced levels of MCP-1 mRNA transcripts with IC50 of 75 µM. It inhibits the production of MCP-1 by LPS-stimulated MM6 cells with IC50 of 425 μM, without affecting the release of IL-8 or IL-6. This chemical inhibits the release of MCP-1 from IL-1 stimulated osteoblast cell line Saos-2. It, even at the maximal concentration, does not exhibit a direct in vitro cytotoxic effect on human IIB-MEL-J melanoma or ECs, although it inhibits MCP-1 expression. This compound (10-300 μM) reduces rat vascular smooth muscle cell (VSMC) proliferation, migration, and invasion. It induces the downregulation of the classical NF-κB pathway. This chemical displays a specific inhibitory effect on the p65 and p65/p50 induced MCP-1 promoter activation, with no effect on other tested activated promoters, indicating that it acts on a specific subpopulation of NF-κB isoforms and selects its targets within the whole NF-κB inflammatory pathway. It modulates cancer-cell proliferation and migration, mainly through negative regulation of TGF-β and AKT signaling. |
| In vivo |
Oral administration of Bindarit at 50 mg/kg in NZB/W mice delays the onset of proteinuria, significantly protects from renal function impairment, and prolongs survival of NZB/W mice or lupus mice. This compound treatment completely MCP-1 up-regulation during the progression of nephritis. Inhibition of MCP-1 with this chemical also reduces tumor growth and macrophage recruitment, rendering necrotic tumor masses in human melanoma xenografts. This compound is effective in reducing neointima formation in both non-hyperlipidaemic and hyperlipidaemic animal models of vascular injury by a direct effect on VSMC proliferation and migration and by reducing neointimal macrophage content. Administration of this chemical results in impaired metastatic disease in prostate cancer PC-3M-Luc2 xenograft mice and impairment of local tumorigenesis in Balb/c mice with murine breast cancer 4T1-Luc cells. In addition, this treatment significantly decreases the infiltration of tumor-associated macrophages and myeloid-derived suppressor cells in 4T1-Luc primary tumors. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01269242 | Completed | Coronary Restenosis |
Aziende Chimiche Riunite Angelini Francesco S.p.A |
January 2009 | Phase 2 |
| NCT01109212 | Completed | Diabetic Nephropathy |
Aziende Chimiche Riunite Angelini Francesco S.p.A|Mario Negri Institute for Pharmacological Research |
March 2007 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































