research use only
Mifepristone (RU-486) Progesterone Receptor Antagonist
Cat.No.S2606
Chemical Structure
Molecular Weight: 429.59
Quality Control
| Related Targets | Adrenergic Receptor GPR Androgen Receptor Glucocorticoid Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES THR |
|---|---|
| Other Estrogen/progestogen Receptor Inhibitors | Elacestrant (RAD1901) Dihydrochloride Vepdegestrant (ARV-471) MPP dihydrochloride Kaempferol Cholesterol G15 Endoxifen HCl Chrysin Licochalcone A AZD9496 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CHO-K1 cells | Function assay | Inhibition of CHO-K1 cells expressing glucocorticoid receptor, IC50=8e-06 μM | ||||
| T47D-C124 cells | Function assay | 24 h | Antagonist activity at progesterone receptor in human T47D-C124 cells transfected with luciferase gene linked to MMTV promoter assessed as inhibition of progesterone-induced luciferase transactivation activity after 24 hrs, IC50=2.1e-05 μM | |||
| neuroblastoma cells | Function assay | In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor, IC50=2.5e-05 μM | ||||
| T47D cells | Function assay | 48 h | Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity after 48 hrs, IC50=4.5e-05 μM | |||
| CV-1 cells | Function assay | Antagonistic activity against human progesterone receptor B (hPR-B) in co-transfected CV-1 cells, IC50=0.00018 μM | ||||
| HEK293 cells | Function assay | Antagonist activity against glucocorticoid receptor (unknown origin) expressed in HEK293 cells by GRE-dependent luciferase reporter gene assay, IC50=0.000298 μM | ||||
| COS7 cells | Function assay | Antagonist activity at cloned glucocorticoid receptor-ligand binding domain expressed in african green monkey COS7 cells by GAL4 luciferase reporter assay, IC50=0.0006 μM | ||||
| SW1353 cells | Function assay | Binding affinity to glucocorticoid receptor in SW1353 cells by whole-cell binding assay, Ki=0.00082 μM | ||||
| A549 cells | Function assay | Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luciferase reporter gene assay, IC50=0.0016 μM | ||||
| A549 cells | Function assay | 16 h | Antagonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of corticoid-induced transcription after 16 hrs by glucocorticoid response element-driven luciferase reporter gene assay, IC50=0.0016 μM | |||
| rat H4-IIE cells | Function assay | 1 h | Antagonist activity against glucocorticoid receptor in rat H4-IIE cells assessed as inhibition of dexamethasone-induced receptor transactivation pre-incubated for 1 hr before dexamethasone addition and measured 24 hrs post dexamethasone stimulation by tyrosine aminotransferase enzyme assay, IC50=0.00194 μM | |||
| HeLa cells | Function assay | Effective concentration against inhibition of Dexamethasone induced glucocorticoid receptor transactivation of mouse mammary tumor virus luciferase gene in HeLa cells, EC50=0.002 μM | ||||
| NIH3T3 cells | Function assay | In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor, IC50=0.0022 μM | ||||
| CHO cells | Function assay | Inhibition of Dexamethasone stimulated transcriptional activity in CHO cells expressing glucocorticoid receptor, IC50=0.005 μM | ||||
| hGRAF cells | Function assay | Inhibition of human GR expressed in hGRAF cells, Ki=0.005 μM | ||||
| COS-1 | Function assay | Binding affinity for human androgen receptor in transiently-transfected COS-1 cells, Ki=0.022 μM | ||||
| rat hepatocytes | Function assay | Inhibition of dexamethasone-induced GR-mediated tyrosine amino transferase activity in rat hepatocytes, IC50=0.27 μM | ||||
| human K562/R7 cells | Function assay | 72 h | Potentiation of doxorubicin-induced cytotoxicity against doxorubicin-resistant human K562/R7 cells assessed as doxorubicin IC50 at 1 uM after 72 hrs by MTT assay, IC50=0.9 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 85 mg/mL
(197.86 mM)
Ethanol : 85 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 429.59 | Formula | C29H35NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 84371-65-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | C-1073, RU 38486 | Smiles | CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O | ||
Mechanism of Action
| Features |
Mifepristone is the first approved medication for patients with endogenous cushing
|
|---|---|
| Targets/IC50/Ki |
Bcl-2
Progesterone receptor
(T47D cells) 0.2 nM
Glucocorticoid receptor
(A549 cells) 2.6 nM
|
| In vitro |
Mifepristone inhibit corticoid-induced transcription from a glucocorticoid response element (GRE)-linked luciferase reporter gene in the human lung carcinoma cell line A549. Moreover, this compound also blocks progesterone induction of alkaline phosphatase activity in the human breast cancer cell line T47D. It inhibits ovarian cancer cell growth of SK-OV-3 and OV2008 with IC50 of 6.25 μM and 6.91 μM, respectively. A recent study shows that this chemical induces caspase-1 over expression both in differentiated and undifferentiated caspase-1-embryonic stem cells. |
| Kinase Assay |
Glucocorticoid receptor (GR) antagonist activity, Progesterone receptor (PR) antagonist activity
|
|
T47D alkaline phosphatase assay: T47D human breast cancer cells are plated in 96-well tissue culture plates at 104 cells per well in assay medium [RPMI medium without phenol red containing 5% (v/v) charcoal-treated FBS and 1% (v/v) penicillin–streptomycin]. Two days later, the medium is decanted and Mifepristone or control is added at a final concentration in fresh assay medium. Twenty-four hours later, an alkaline phosphatase assay is performed using a SEAP kit. The medium is decanted and the cells are fixed for 30 minutes at room temperature with 5% (v/v) formalin. The cells are washed once at room temperature with Hanks
|
|
| In vivo |
Mifepristone can impair the growth of SK-OV-3 tumors in immunosuppressed mice at 0.5 mg/day and 1 mg/day. This compound inhibits the prostate weight significantly in the highest doses in vivo, and inhibits growth of the prostate gland produced by dihydrotestosterone (DHT) to a greater extent than the induction of atrophy and cell death in rats. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
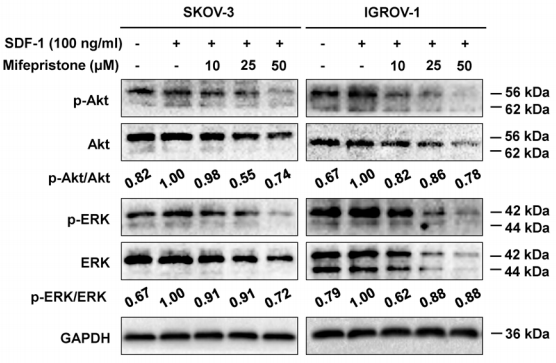
| Western blot | p-AKT / AKT / p-ERK / ERK MMP-2 / MMP-9 / COX-2 / VEGF |
|
28938623 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06394999 | Not yet recruiting | Female Contraception |
Leiden University Medical Center|Karolinska Institutet|Women on Waves|Children''s Investment Fund Foundation |
September 2024 | Phase 3 |
| NCT05177510 | Recruiting | Labor Induced |
Chelsea and Westminster NHS Foundation Trust |
August 25 2023 | Phase 3 |
| NCT04905251 | Recruiting | Medical Abortion |
Linepharma International LTD |
February 22 2022 | -- |
| NCT05062174 | Withdrawn | BRCA1 Mutation|High-grade Serous Ovarian Cancer|TNBC - Triple-Negative Breast Cancer |
Indiana University|Breast Cancer Research Foundation |
November 1 2021 | -- |
| NCT04588688 | Terminated | Central Adrenal Insufficiency|Mifepristone |
Tobias Else|Corcept Therapeutics|University of Michigan |
May 5 2021 | Phase 2 |
| NCT03659045 | Completed | Abortion-Related Disorders |
Assistance Publique Hopitaux De Marseille |
January 15 2019 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































