- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Brilanestrant (GDC-0810) Estrogen/progestogen Receptor antagonist
Cat.No.S7855
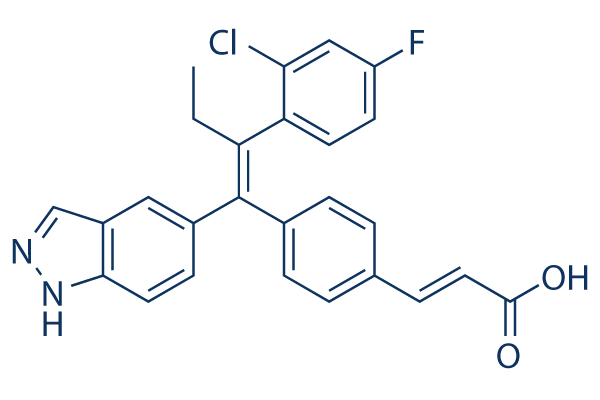
Chemical Structure
Molecular Weight: 446.90
Jump to
Quality Control
| Related Targets | Adrenergic Receptor GPR Androgen Receptor Glucocorticoid Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES THR |
|---|---|
| Other Estrogen/progestogen Receptor Products | Elacestrant (RAD1901) Dihydrochloride Vepdegestrant (ARV471) MPP dihydrochloride Cholesterol Endoxifen HCl G15 Chrysin Licochalcone A AZD9496 PHTPP |
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(199.14 mM)
Ethanol : 89 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 446.90 | Formula | C26H20ClFN2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1365888-06-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | ARN-810 | Smiles | CCC(=C(C1=CC=C(C=C1)C=CC(=O)O)C2=CC3=C(C=C2)NN=C3)C4=C(C=C(C=C4)F)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
ERα
(Cell-free) 6.1 nM
ERβ
(Cell-free) 8.8 nM
|
|---|---|
| In vitro |
In cell-free radio-ligand competitive binding assays, Brilanestrant (GDC-0810) binds both ERα and ERβ with low nanomolar affinity. This compound has little to no inhibition against CYP1A2, CYP2D6, or CYP3A4 (IC50 > 20 μM), modest inhibitory effect on CYP2C9 and CYP2C19 (IC50 = 2.2 and 3.3 μM respectively), and potent inhibition of CYP2C8 (IC50 of <0.1 μM). Selectivity of it over other nuclear hormone receptors is also found to be good. In transcriptional reporter assays for the mineralocorticoid (MR), progesterone-A (PR-A), progesterone (PR-B), and glucocorticoid (GR) receptors, it has minimal activity (IC50 > 1 μM). While in binding assays, it displays little activity toward the androgen receptor (AR; IC50 > 4 μM) and GR (IC50 = 0.99 μM). GDC-0810-mediated ERα depletion is dependent on the 26S proteasome. It antagonizes ERα ligand binding domain mutants in vitro and in vivo. In cell-free E2 competitive binding assays that was used to determine the binding of this compound to ER.WT, ER.Y537S and ER.D538G ligand binding domains, it retains its ability to potently displace E2 from the ligand binding domain, albeit with a slightly increased IC50 (WT: 2.6 nM vs. ER.Y537S: 5.5 nM and ER.D538G: 5.4 nM). It can compete the PGC1α co-activator peptide off the mutated ligand binding domain, implying that it is capable of driving an 'active' to 'inactive' conformational shift of mutant ER, though with a ~five-seven fold reduction in biochemical potency compared to wild-type ER.
|
| In vivo |
Brilanestrant (GDC-0810) exhibits a pharmacokinetic profile characterized by low clearance across species and good bioavailability (40–60%). As would be expected for a lipophilic carboxylic acid, the compound is highly bound to plasma proteins (>99.5% across species) and has a low to moderate volume of distribution (Vss = 0.2–2.0 L/kg across species). It demonstrates good bioavailability across species and displays robust activity in tamoxifen-sensitive and tamoxifen-resistant xenograft models of breast cancer. This compound also exhibits mild estrogenic activity in uterine models in vitro and in vivo.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02802670 | Completed | Healthy Volunteers |
Genentech Inc. |
May 2016 | Phase 1 |
| NCT02621957 | Completed | Breast Cancer |
Genentech Inc. |
December 2015 | Phase 1 |
| NCT02569801 | Terminated | Breast Cancer |
Genentech Inc. |
December 4 2015 | Phase 2 |
| NCT01823835 | Terminated | Breast Cancer |
Genentech Inc. |
December 29 2014 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































