- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Bimiralisib (PQR309) PI3K inhibitor
Cat.No.S8738
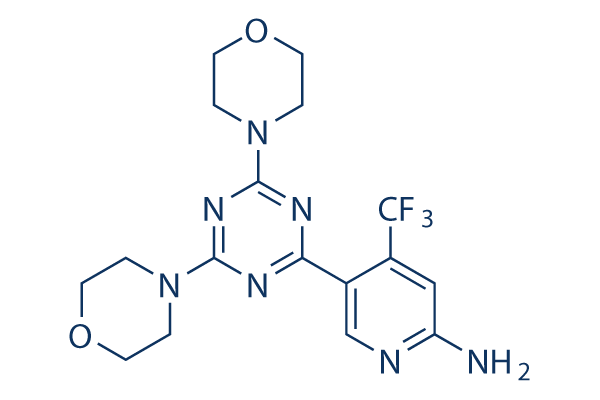
Chemical Structure
Molecular Weight: 411.38
Jump to
Quality Control
Batch:
Purity:
99.02%
99.02
Solubility
|
In vitro |
DMSO
: 6 mg/mL
(14.58 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 411.38 | Formula | C17H20F3N7O2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1225037-39-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1COCCN1C2=NC(=NC(=N2)C3=CN=C(C=C3C(F)(F)F)N)N4CCOCC4 | ||
Mechanism of Action
| Targets/IC50/Ki |
PI3Kα
(Cell-free assay) 1.5 nM(Kd)
PI3Kβ
(Cell-free assay) 11 nM(Kd)
mTOR
(Cell-free assay) 12 nM(Kd)
PI3Kγ
(Cell-free assay) 25 nM(Kd)
PI3Kδ
(Cell-free assay) 25 nM(Kd)
|
|---|---|
| In vitro |
Bimiralisib (PQR309) shows in vitro activity with a median IC50 value of 233 nmol/L (95% CI, 174-324 nmol/L) in most of the tesed lymphoma cell lines (increasing doses, 72 hours). The arrest in proliferation is mainly due to cell cycle arrest with a block in G1 rather than to apoptosis, limited to only 2/7 cell lines. It is more active in B-cell lymphoma cell lines (DLBCL, MCL, CLL, and SMZL) than in the T-cell derived ALCL. This compound inhibits PI3K/mTOR signaling in lymphoma cell lines. It has in vitro and in vivo antilymphoma activity as single agent and in combination. |
| In vivo |
Bimiralisib (PQR309) is orally available, crosses the blood−brain barrier, and displayed favorable pharmacokinetic parameters in mice, rats, and dogs. It shows little clearance when exposed to rat, dog, and human liver microsomes, with a quicker turnover in mouse liver microsomes, where 40% of the compound was eliminated within 30 min. In female mice, plasma concentrations depended on the drug administration route, resulting in half-lives of approximately 13-36 min for po administration vs 9-10 min for iv administration. This compound shows excellent oral bioavailability (>50%). Male Beagle dogs, exposed to it at 10 mg/kg po, showed maximal drug plasma concentrations Cmax of 583 ng/mL (approximately 1.5 μM) after 60-90 min and a half-life of >7 h, which results in drug levels of approximately 0.38 μM (150 ng/mL) after 24 h. The oral bioavailability in male Beagle dogs was estimated to be 23%. Altogether the PK studies in the three models (female CD-1 mouse, female Sprague-Dawley rats, male Beagle dog) show rapid absorption and good oral bioavailability. It demonstrates efficiency in inhibiting proliferation in tumor cell lines (PC3 prostate cancer cells) and a rat xenograft model (PC3 xenograft model). |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02483858 | Completed | Cancer |
PIQUR Therapeutics AG|Roswell Park Cancer Institute|M.D. Anderson Cancer Center|Mayo Clinic|Hospital Clinic of Barcelona|University College London Hospitals|Churchill Hospital|Case Western Reserve University|University Hospital Zürich |
March 21 2019 | Phase 1 |
| NCT03127020 | Completed | Lymphoma|Non-Hodgkin Lymphoma |
PIQUR Therapeutics AG|University Hospital Basel Switzerland|University Hospital Munich|University Hospital Freiburg|Charite University Berlin Germany|University of Stuttgart |
June 2016 | Phase 2 |
| NCT02723877 | Completed | Metastatic Breast Cancer |
PIQUR Therapeutics AG|Hospital Universitario Ramon y Cajal|Hospital Universitari Vall d''Hebron Research Institute|Institut Català d''Oncologia|Churchill Hospital|Barts Cancer Institute|Fundación Instituto Valenciano de Oncología |
March 28 2016 | Phase 1|Phase 2 |
| NCT02850744 | Terminated | Glioblastoma Multiforme |
PIQUR Therapeutics AG|University Hospital Basel Switzerland|Insel Gruppe AG University Hospital Bern|University Hospital Zürich |
July 2015 | Phase 2 |
| NCT02249429 | Completed | Lymphoma Malignant |
PIQUR Therapeutics AG|University College London Hospitals|Churchill Hospital|Royal Marsden NHS Foundation Trust|University of Haifa|Weill Medical College of Cornell University|Institut Curie|University Clinical Center Sarajevo|Clinical Center Kragujevac|Clinical Center Nis Nis|Institute for Oncology and Radiology Serbia Belgrade|University Clinical Centre of Republic of Srpska |
May 2015 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































