research use only
Gedatolisib (PKI-587) PI3K inhibitor
Cat.No.S2628
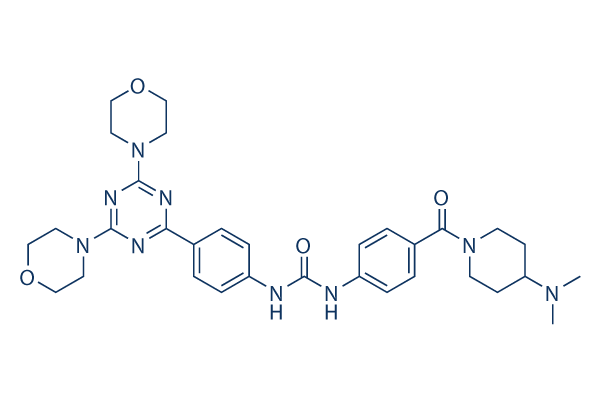
Chemical Structure
Molecular Weight: 615.73
Quality Control
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MDA-MB-361 cells | Cytotoxicity assay | Cytotoxicity against human MDA-MB-361 cells, IC50=0.004 μM | ||||
| PC3MM2 cells | Cytotoxicity assay | Cytotoxicity against human PC3MM2 cells, IC50=0.0131 μM | ||||
| MDA-MB-361 cells | Function assay | Inhibition of Akt T308 phosphorylation in human MDA-MB-361 cells by Western blotting, IC50=0.008 μM | ||||
| MDA-MB-361 cells | Function assay | Inhibition of Akt S473 phosphorylation in human MDA-MB-361 cells by Western blotting, IC50=0.01 μM | ||||
| MDA-MB-361 cells | Function assay | Inhibition of eNOS phosphorylation in human MDA-MB-361 cells by Western blotting | ||||
| MDA-MB-361 cells | Function assay | Inhibition of PARS40 phosphorylation in human MDA-MB-361 cells by Western blotting | ||||
| MDA-MB-361 cells | Function assay | Inhibition of GSK3 kinase phosphorylation in human MDA-MB-361 cells by Western blotting | ||||
| MDA-MB-361 cells | Function assay | Inhibition of mTOR TORC1 kinase activity in human MDA-MB-361 cells assessed as suppression of p70S6K phosphorylation at < 30 nM | ||||
| MDA-MB-361 cells | Function assay | Inhibition of mTOR TORC1 kinase activity in human MDA-MB-361 cells assessed as suppression of 4EBP1 phosphorylation at < 30 nM | ||||
| MDA-MB-361 cells | Function assay | Inhibition of Akt T308 phosphorylation in human MDA-MB-361 cells xenografted mouse model upto 36 hrs | ||||
| MDA-MB-361 cells | Function assay | Inhibition of Akt S473 phosphorylation in human MDA-MB-361 cells xenografted mouse model upto 36 hrs | ||||
| MDA-MB-361 cells | Function assay | Induction of PARP cleavage in human MDA-MB-361 cells xenografted mouse model upto 18 hrs | ||||
| MDA-MB-361 cells | Function assay | Antitumor activity against human MDA-MB-361 cells xenografted mouse model assessed as reduction in tumor volume at 20 mg/kg, iv on day 1, 5, 9 | ||||
| SF9 insect cells | Function assay | 2 h | Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay, IC50=0.0004 μM | |||
| SF9 insect cells | Function assay | 2 h | Inhibition of human PI3Kgamma expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay, IC50=0.011μM | |||
| MDA-MB-361 cells | Growth inhibition assay | 72 h | Growth inhibition of human MDA-MB-361 cells after 72 hrs, IC50=0.003 μM | |||
| human PC3 cells | Growth inhibition assay | 72 h | Growth inhibition of human PC3 cells after 72 hrs, IC50=0.011 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 3 mg/mL
(4.87 mM)
Ethanol : 1 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 615.73 | Formula | C32H41N9O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1197160-78-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | PF-05212384 | Smiles | CN(C)C1CCN(CC1)C(=O)C2=CC=C(C=C2)NC(=O)NC3=CC=C(C=C3)C4=NC(=NC(=N4)N5CCOCC5)N6CCOCC6 | ||
Mechanism of Action
| Targets/IC50/Ki |
PI3Kα
(Cell-free assay) 0.4 nM
mTOR
(Cell-free assay) 1.6 nM
PI3Kγ
(Cell-free assay) 5.4 nM
|
|---|---|
| In vitro |
Gedatolisib (PKI-587) shows potent inhibitory activity against PI3K-α, PI3K-γ and mTOR with IC50 of 0.4 nM, 5.4 nM and 1.6 nM, respectively. Furthermore, it also exhibits its potency against the most frequently occurring mutant forms of PI3Kα, notably the H1047R and E545K with IC50 of 0.6 nM and 0.6 nM, respectively. Correlated with suppression of phosphorylation of PI3K/mTOR signaling pathway proteins, this compound causes tumor cell growth inhibition in MDA-361 and PC3-MM2 cell lines with IC50 of 4 nM and 13.1 nM, respectively.
|
| Kinase Assay |
PI3K and mTOR kinase assay
|
|
Enzyme assays are done in fluorescent polarization (FP) format, adapted from the Echelon K-1100 PI3K FP assay kit protocol. Human class I PI3Ks and PI3K-α mutants (E545K and H1047R) are produced in Sf9 or purchased from Upstate Biotech. GST-GRP1 (murine) is produced in Escherichia coli and isolated by GST-Sepharose. Assay buffers are reaction buffer [20 mM HEPES (pH 7.1), 2 mM MgCl2, 0.05% CHAPS, and 0.01% β-mercaptoethanol] and stop/detection buffer [100 mM HEPES (pH 7.5), 4 mM EDTA, 0.05% CHAPS]. FP reaction is run for 30 minutes at room temperature in 20 μL of reaction buffer containing 20 μM phosphatidylinositol 4,5-bisphosphate (PIP2), 25 μM ATP, and <4% DMSO. FP reaction is stopped with 20 μL of stop/detection buffer (10 nM probe and 40 nM GST-GRP), and after 2 hours, data are collected using an Envision plate reader. The routine assays with purified FLAG-TOR (FL and 3.5) are performed in 96-well plates as follows. Enzymes are first diluted in kinase assay buffer (10 mM Hepes (pH 7.4), 50 mM NaCl, 50 mM β-glycerophosphate, 10 mM MnCl2, 0.5 mM DTT, 0.25 μM microcystin LR, and 100 μg/mL BSA). To each well, 12 μL of the diluted enzyme is mixed briefly with 0.5 μL test inhibitor or control vehicle dimethyl sulfoxide (DMSO), where the test inhibitor is Gedatolisib (PKI-587). The kinase reaction is initiated by adding 12.5 μL kinase assay buffer containing ATP and His6-S6K to give a final reaction volume of 25 μL containing 800 ng/mL FLAG-TOR, 100 μM ATP, and 1.25 μM His6-S6K. The reaction plate is incubated for 2 hours (linear at 1–6 hours) at room temperature with gentle shaking and then terminated by adding 25 μL Stop buffer (20 mM Hepes (pH 7.4), 20 mM EDTA, and 20 mM EGTA).
|
|
| In vivo |
Gedatolisib (PKI-587) treatment at 25 mg/kg iv in nude mice leads to low plasma clearance (7 (mL/min)/kg), high volume of distribution (7.2 L/kg), and long half-life (14.4 hours). In the MDA-361 xenograft model, it produces potent antitumor efficacy with the minimum efficacious dose (MED) of 3 mg/kg against MDA-361 tumors and maximum tolerated single dose (MTD) of 30 mg/kg. While in the H1975 (non-small-cell lung carcinoma, mutant EGFR [L858R, T790M]) xenograft model, this compound at 25 mg/kg for 7 weeks results in 90% survival of the group treated.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
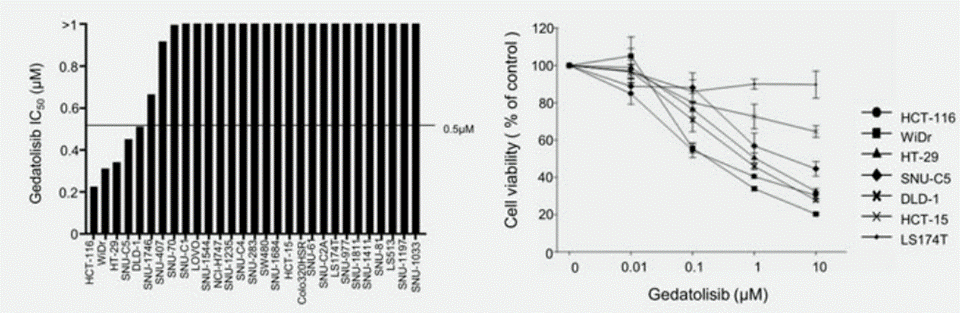
|
29978469 |
| Western blot | p-AKT / p-mTOR / p-p70S6K / p-S6K / p-4E-BP1 / AKT / mTOR / p70S6K / S6K / 4EBP1 TSC1 / TSC2 / Raptor |
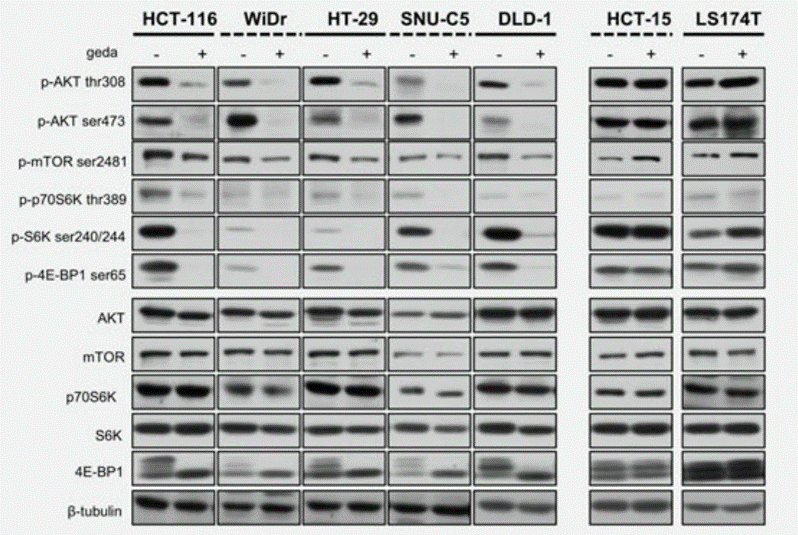
|
29978469 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02438761 | Terminated | Therapy-related Acute Myeloid Leukemia and Myelodysplastic Syndrome|Acute Myeloid Leukemia in Relapse|de Novo Acute Myeloid Leukemia at Diagnostic |
Institut Curie|Fondation ARC|National Cancer Institute France |
August 31 2015 | Phase 2 |
| NCT02069158 | Completed | Breast Cancer|NSCLC|Ovary Cancer|Endometrial Cancer|Small Cell Lung Cancer (SCLC)|Head and Neck (HNSCC) |
Cristiana Sessa|Oncology Institute of Southern Switzerland |
April 2014 | Phase 1 |
| NCT01420081 | Terminated | Endometrial Neoplasms |
Pfizer |
January 19 2012 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































