research use only
ZSTK474 PI3K inhibitor
Cat.No.S1072
Chemical Structure
Molecular Weight: 417.41
Quality Control
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Sf21 insect cells | Function assay | 1 h | Inhibition of bovine recombinant PI3K p110delta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging, IC50=0.7 nM | |||
| human HCT116 cells | Function assay | 15 mins | Inhibition of PIK3CA H1047R mutant-mediated cell signaling in human HCT116 cells expressing PTEN assessed as inhibition of insulin-induced pAkt/PKB phosphorylation at Thr308 treated for 15 mins before insulin challenge measured after 5 mins by immunoblotting, IC50=78 nM | |||
| human LNCAP cells | Proliferation assay | 3 days | Antiproliferative activity against human LNCAP cells after 3 days by MTS assay, IC50=0.21 μM | |||
| human NZB5 cells | Proliferation assay | 5 days | Antiproliferative activity against human NZB5 cells expressing wild type p110alpha assessed as incorporation of [3H]thymidine after 5 days, IC50=0.22 μM | |||
| human NZOV9 cells | Proliferation assay | 5 days | Antiproliferative activity against human NZOV9 cells expressing p110alpha kinase Y1021C mutant assessed as incorporation of [3H]thymidine after 5 days, IC50=0.29 μM | |||
| human MDA-MB-468 cells | Cytotoxic assay | 48 h | Cytotoxicity against PTEN-deficient human MDA-MB-468 cells assessed as inhibition of cell growth after 48 hrs by Cell Titer 96 assay | |||
| human A549 cells | Function assay | 10 μM | 1 h | Inhibition of PI3K in human A549 cells assessed as reduction in pAkt level at 10 uM after 1 hr by Western blotting analysis | ||
| human DMS114 cells | Growth inhibition assay | Growth inhibition of human DMS114 cells | ||||
| human MKN74 cells | Growth inhibition assay | Growth inhibition of human MKN74 cells | ||||
| human SNB78 cells | Growth inhibition assay | Growth inhibition of human SNB78 cells | ||||
| human St-4 cells | Growth inhibition assay | Growth inhibition of human St-4 cells | ||||
| human DU145 cells | Growth inhibition assay | Growth inhibition of human DU145 cells | ||||
| human LOXIMVI cells | Growth inhibition assay | Growth inhibition of human LOXIMVI cells | ||||
| human PC3 cells | Growth inhibition assay | Growth inhibition of human PC3 cells | ||||
| human LOXIMVI cells | Growth inhibition assay | Growth inhibition of human LOXIMVI cells | ||||
| human OVCAR3 cells | Growth inhibition assay | Growth inhibition of human OVCAR3 cells | ||||
| human SKOV3 cells | Growth inhibition assay | Growth inhibition of human SKOV3 cells | ||||
| human KM12 cells | Growth inhibition assay | Growth inhibition of human KM12 cells | ||||
| human HT-29 cells | Growth inhibition assay | Growth inhibition of human HT-29 cells | ||||
| human HCT15 cells | Growth inhibition assay | Growth inhibition of human HCT15 cells | ||||
| human NCI-H226 cells | Growth inhibition assay | Growth inhibition of human NCI-H226 cells | ||||
| human NCI-H522 cells | Growth inhibition assay | Growth inhibition of human NCI-H522 cells | ||||
| human A549 cells | Growth inhibition assay | Growth inhibition of human A549 cells | ||||
| human HCC2998 cells | Growth inhibition assay | Growth inhibition of human HCC2998 cells | ||||
| human SNB75 cells | Growth inhibition assay | Growth inhibition of human SNB75 cells | ||||
| human OVCAR4 cells | Growth inhibition assay | Growth inhibition of human OVCAR4 cells | ||||
| human OVCAR5 cells | Growth inhibition assay | Growth inhibition of human OVCAR5 cells | ||||
| human OVCAR8 cells | Growth inhibition assay | Growth inhibition of human OVCAR8 cells | ||||
| human SKOV3 cells | Growth inhibition assay | Growth inhibition of human SKOV3 cells | ||||
| human ACHN cells | Growth inhibition assay | Growth inhibition of human ACHN cells | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 15 mg/mL
(35.93 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 417.41 | Formula | C19H21F2N7O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 475110-96-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1COCCN1C2=NC(=NC(=N2)N3C4=CC=CC=C4N=C3C(F)F)N5CCOCC5 | ||
Mechanism of Action
| Features |
First orally administered PI3K inhibitor used in vivo.
|
|---|---|
| Targets/IC50/Ki |
PI3Kδ
(Cell-free assay) 4.6 nM
PI3Kα
(Cell-free assay) 16 nM
PI3K
(Cell-free assay) 37 nM
PI3Kβ
(Cell-free assay) 44 nM
PI3Kγ
(Cell-free assay) 49 nM
|
| In vitro |
ZSTK474 at 1 μM potently reduces PI3K activity to 4.7% of the control level, whereas LY2194002 only reduces the activity to 44.6% of the control. This compound inhibits the activities of recombinant p110β, -γ, and -δ with IC50 of 17 nM, 53 nM, and 6 nM, respectively. It shows potent antiproliferative activity against a panel of 39 human cancer cell lines with mean GI50 of 0.32 μM, more effectively than that of LY294002 or wortmannin with mean GI50 of 7.4 μM or 10 μM, respectively. This chemical treatment at 1 μM blocks membrane ruffling and generation of PIP3 induced by platelet-derived growth factor in murine embryonic fibroblasts (MEFs). It at 10 μM induces apoptosis in OVCAR3 cells, and induces complete G1-phase arrest but not apoptosis in A549 cells. This compound treatment at 0.5 μM significantly decreases the level of phosphorylated Akt and GSK-3β, as well as the cyclin D1 protein expression. It also inhibits the phosphorylation of other downstream signaling components that are involved in regulating cell proliferation including FKHRL1, FKHR, TSC-2, mTOR, and p70S6K in a dose-dependent manner. This chemical does not inhibit mTOR at 0.1 μM, and even at a concentration of 100 μM, it inhibits mTOR activity less than 40%. It blocks VEGF-induced cell migration and the tube formation in human umbilical vein endothelial cells (HUVECs), and inhibits the expression of HIF-1α and secretion of VEGF in RXF-631L cells, exhibiting potent in vitro antiangiogenic activity. This compound treatment inhibits the production of IFNγ and IL-17 in concanavalin A-activated T cells, and inhibits the proliferation and PGE(2) production by fibroblast-like synovial cells (FLS). |
| Kinase Assay |
Inhibition of PI3K activity
|
|
A549 cells are lysed in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, and 1% Igepal CA-630, the lysates are centrifuged at 20,000 g and 4 °C for 10 minutes, and the supernatants are used as cell lysate (protein = 2-4 mg/mL). To immunoprecipitate PI3K, 200 μL of cell lysate are incubated with anti-p85 polyclonal antibody and protein G-agarose (5 μL). PI3Kα, PI3Kβ, and PI3Kδ can be immunoprecipitated by the anti-p85 polyclonal antibody. Agarose beads containing immunoprecipitates are washed twice with buffer A (20 mM Tris-HCl at pH 7.5, 150 mM NaCl, 5 mM EDTA, and 1% Igepal CA-630), once with buffer B (500 mM LiCl and 100 mM Tris-HCl at pH 7.5), once with distilled water, and once with buffer C (100 mM NaCl and 20 mM Tris-HCl at pH 7.5). Immunoprecipitates are suspended in 20 μL of buffer C containing phosphatidylinositol of 200 μg/mL. The mixture is preincubated with increasing concentrations of this compound at 25 °C for 5 minutes. [γ-32P]ATP (2 μCi per assay mixture; final concentration, 20 μM) and MgCl2 (final concentration, 20 mM) are added to start the reaction. The reaction mixture is incubated at 25 °C for 20 minutes. Phosphorylated products of phosphatidylinositol are separated by thin-layer chromatography and visualized by autoradiography. The phosphatidylinositol-3-phosphate region is scraped from the plate, and radioactivity is also measured with liquid scintillation spectroscopy. The level of inhibition for this chemical is determined as the percentage of 32P counts per minute obtained without this compound.
|
|
| In vivo |
Oral administration of ZSTK474 inhibits the growth of subcutaneously implanted mouse B16F10 melanoma tumors in a dose-dependent manner, producing tumor regression of 28.5%, 7.1%, or 4.9% on day 14 at 100, 200, or 400 mg/kg, respectively, which is superior to that of the four major anticancer drugs at their respective maximum tolerable doses with tumor regression of 96%, 35.7%, 24%, or 68.3%, respectively. This compound treatment at 400 mg/kg completely inhibits the growth of A549, PC-3, and WiDr xenografts in mice, and induces the regression of A549 xenograft tumors. It significantly inhibits tumor growth in the RXF-631L xenograft model, correlated with a significantly reduced number of microvessels in the treated mice. Oral administration of this chemical ameliorates the progression of adjuvant-induced arthritis (AIA) in rats.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
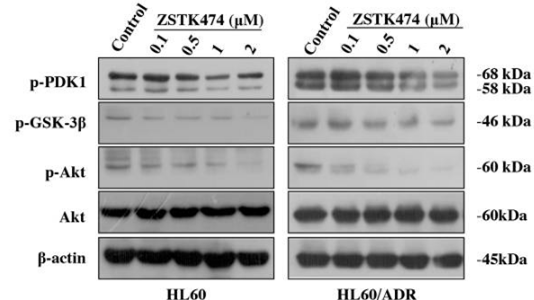
| Western blot | p-PDK1 / p-GSK3β / p-AKT / AKT P-gp / MRP1 p-Rb / p27 / Cyclin D1 HIF-1α / HIF-1β |
|
28388564 |
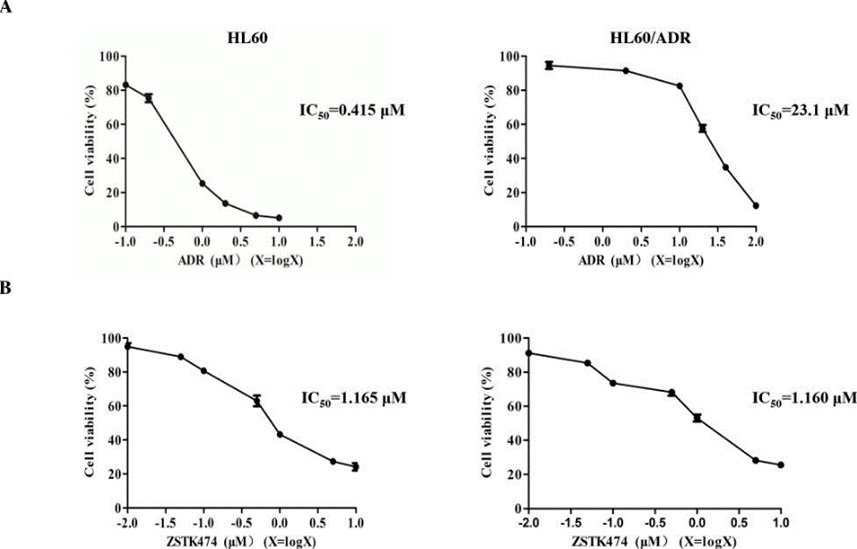
| Growth inhibition assay | Cell viability |
|
28388564 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































