- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Eganelisib (IPI-549) PI3Kγ Inhibitor
Cat.No.S8330
Chemical Structure
Molecular Weight: 528.56
Jump to
Quality Control
Batch:
Purity:
99.98%
99.98
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | LY294002 Buparlisib (BKM120) SAR405 XL147 analogue Paxalisib (GDC-0084) Tersolisib (STX-478) 3-Methyladenine (3-MA) Dactolisib (BEZ235) Pictilisib (GDC-0941) Wortmannin (SL-2052) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Sf9 | Function assay | Binding affinity to human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells by equilibrium fluorescence titration analysis, Kd = 0.00029 μM. | 27660692 | |||
| RAW264.7 | Function assay | 30 mins | Inhibition of PI3Kgamma in C5a-stimulated mouse RAW264.7 cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by stimulation with C5a for 3 mins by ELISA, IC50 = 0.0012 μM. | 27660692 | ||
| Sf9 | Function assay | 15 mins | Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 15 mins followed by substrate addition measured after 2 hr by ADP-Glo luminescence assay, IC50 = 0.016 μM. | 27660692 | ||
| Sf9 | Function assay | Binding affinity to human recombinant full length N-terminal His6-tagged PI3K p110alpha/p85alpha coexpressed in baculovirus infected Sf9 insect cells by equilibrium fluorescence titration analysis, Kd = 0.017 μM. | 27660692 | |||
| Sf9 | Function assay | Binding affinity to human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells by equilibrium fluorescence titration analysis, Kd = 0.023 μM. | 27660692 | |||
| Sf9 | Function assay | Binding affinity to human recombinant full length N-terminal His6-tagged PI3K p110beta/p85alpha coexpressed in baculovirus infected Sf9 insect cells by equilibrium fluorescence titration analysis, Kd = 0.082 μM. | 27660692 | |||
| Raji | Function assay | 30 mins | Inhibition of PI3Kdelta in IgM-stimulated human Raji cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by stimulation with IgM for 30 mins by ELISA, IC50 = 0.18 μM. | 27660692 | ||
| 786-O | Function assay | 30 mins | Inhibition of PI3Kbeta in human 786-O cells assessed as reduction in AKT phosphorylation at S473 after 30 mins by ELISA, IC50 = 0.24 μM. | 27660692 | ||
| SKOV-3 | Function assay | 30 mins | Inhibition of PI3Kalpha in human SKOV-3 cells assessed as reduction in AKT phosphorylation at S473 after 30 mins by ELISA, IC50 = 0.25 μM. | 27660692 | ||
| Sf9 | Function assay | 15 mins | Inhibition of human recombinant full length N-terminal His6-tagged PI3K p110alpha/p85alpha coexpressed in baculovirus infected Sf9 insect cells using diC8PIP2 as substrate incubated for 15 mins followed by substrate addition measured after 2 hr by ADP-Glo, IC50 = 3.2 μM. | 27660692 | ||
| Sf9 | Function assay | 15 mins | Inhibition of human recombinant full length N-terminal His6-tagged PI3K p110beta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using diC8PIP2 as substrate incubated for 15 mins followed by substrate addition measured after 2 hr by ADP-Glo , IC50 = 3.5 μM. | 27660692 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(189.19 mM)
Water : ˂1 mg/mL Ethanol : ˂1 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 528.56 | Formula | C30H24N8O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1693758-51-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C1=CC2=C(C(=CC=C2)C#CC3=CN(N=C3)C)C(=O)N1C4=CC=CC=C4)NC(=O)C5=C6N=CC=CN6N=C5N | ||
Mechanism of Action
| Targets/IC50/Ki |
PI3Kγ
(Cell-free assay) 16 nM
|
|---|---|
| In vitro |
Eganelisib (IPI-549) is found to be a remarkably tight binder to PI3K-γ with a Kd of 290 pM and >58-fold weaker affinity for other Class I PI3K isoforms. It does not significantly inhibit a panel of 468 mutant and nonmutant protein and lipid kinases (including Class II PI3K isoforms) at 1 μM. In PI3K-α, -β, -γ, and -δ dependent cellular phospho-AKT assays, this compound demonstrates excellent PI3K-γ potency (IC50 = 1.2 nM) and selectivity against other Class I PI3K isoforms (>146-fold). Furthermore, it dose dependently inhibits PI3K-γ-dependent bone marrow-derived macrophage (BMDM) migration in vitro. It is also found to be selective against a panel of 80 GPCRs, ion channels, and transporters at 10 μM. In vitro, IPI-549 shows moderate to high cell permeability across Caco-2 cell monolayers, is slowly metabolized in cultured hepatocytes (t1/2 > 360 min), and demonstrates IC50s greater than 20 μM for the CYP isoforms tested (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4).
|
| In vivo |
Eganelisib (IPI-549) has excellent oral bioavailability, low clearance, and distributes into tissues with a mean volume of distribution of 1.2 L/kg in vivo (mice, rats, dog, and monkeys). It has a favorable pharmacokinetic profile to allow potent and selective inhibition of PI3K-γ in vivo. This compound can significantly reduce neutrophil migration in a dose-dependent manner in mouse model when administered orally at all of the tested doses. In addition, it has been shown to inhibit tumor growth in murine syngeneic models through alteration of immune cells in the tumor microenvironment.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-AKT / AKT / p-p65 / p65 |
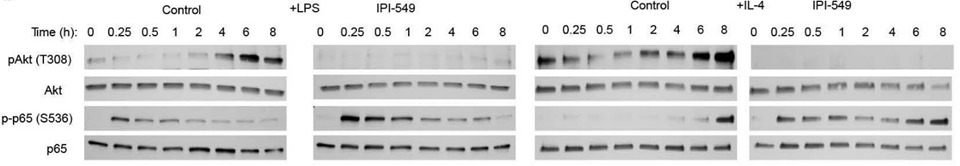
|
27642729 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03980041 | Completed | Bladder Cancer|Urothelial Carcinoma|Solid Tumor|Advanced Cancer |
Infinity Pharmaceuticals Inc.|Bristol-Myers Squibb |
September 25 2019 | Phase 2 |
| NCT02637531 | Unknown status | Advanced Solid Tumors (Part A/B/C/D)|Non-small Cell Lung Cancer (Part E)|Melanoma (Part E)|Squamous Cell Cancer of the Head and Neck (Part E)|Triple Negative Breast Cancer (Part F)|Adrenocortical Carcinoma (Part G)|Mesothelioma (Part G)|High-circulating Myeloid-derived Suppressor Cells (Part H) |
Infinity Pharmaceuticals Inc. |
December 2015 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































