
- Inhibitors
- By product type
- Natural Products
- Inducing Agents
- Peptides
- Antibiotics
- Antibody-drug Conjugates(ADC)
- PROTAC
- Hydrotropic Agents
- Dyes
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
By research - Antibodies
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- Featured Products
- MRTX1133
- Nab-Paclitaxel
- KP-457
- IAG933
- RMC-6236 (Daraxonrasib)
- RMC-7977
- Zoldonrasib (RMC-9805)
- GsMTx4
- Navitoclax (ABT-263)
- TSA (Trichostatin A)
- Y-27632 Dihydrochloride
- SB431542
- SB202190
- MK-2206 Dihydrochloride
- LY294002
- Alisertib (MLN8237)
- XAV-939
- CHIR-99021 (Laduviglusib)
- Bafilomycin A1 (Baf-A1)
- Thiazovivin (TZV)
- CP-673451
- Verteporfin
- DAPT
- Galunisertib (LY2157299)
- MG132
- SBE-β-CD
- Tween 80
- Bavdegalutamide (ARV-110)
- Z-VAD-FMK
- Wnt-C59 (C59)
- IWR-1-endo
- (+)-JQ1
- 3-Deazaneplanocin A (DZNep) Hydrochloride
- RepSox (E-616452)
- Erastin
- Q-VD-Oph
- Puromycin Dihydrochloride
- Cycloheximide
- Telaglenastat (CB-839)
- A-83-01
- Ceralasertib (AZD6738)
- Liproxstatin-1
- Emricasan (IDN-6556)
- PMA (Phorbol 12-myristate 13-acetate)
- Dibutyryl cAMP (Bucladesine) sodium
- Nedisertib (M3814)
- PLX5622
- IKE (Imidazole Ketone Erastin)
- STM2457
- Saruparib (AZD5305)
- New Products
- Contact Us
research use only
Flavopiridol (Alvocidib) CDK inhibitor
Cat.No.S1230
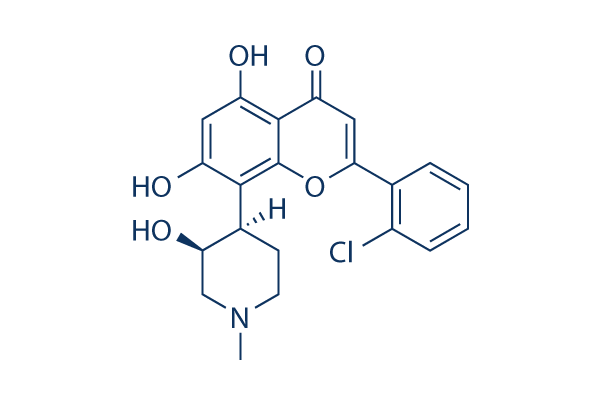
Chemical Structure
Molecular Weight: 401.84
Quality Control
Batch:
Purity:
99.92%
99.92
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MCF-7 tumor cell | Proliferation assay | Inhibition of MCF-7 tumor cell proliferation | 10843211 | |||
| PC3 cell | Function assay | Inhibition of PC3 cell clonogenic assay, IC50=10 μM | 11063609 | |||
| HCT116 cell | Function assay | Inhibition of HCT116 cell clonogenic assay, IC50=13 μM | 11063609 | |||
| A2780 cell | Function assay | Inhibition of A2780 cell clonogenic assay, IC50=15 μM | 11063609 | |||
| Mia PaCa-2 cell | Function assay | Inhibition of Mia PaCa-2 cell clonogenic assay, IC50=36 μM | 11063609 | |||
| LNCaP human prostate carcinoma cell | Proliferation assay | Inhibition of LNCaP human prostate carcinoma cell proliferation | 12190313 | |||
| HCT116/VP35 human colon carcinoma cell | Proliferation assay | Inhibition of HCT116/VP35 human colon carcinoma cell proliferation, IC50=17 nM | 12190313 | |||
| HCT116 human colon carcinoma cell | Proliferation assay | Inhibition of HCT116 human colon carcinoma cell proliferation, IC50=18 nM | 12190313 | |||
| HCT116/VM46 human colon carcinoma cell | Proliferation assay | Inhibition of HCT116/VM46 human colon carcinoma cell proliferation, IC50=21 nM | 12190313 | |||
| A2780/DDP-R human ovarian carcinoma cell | Proliferation assay | Inhibition of A2780/DDP-R human ovarian carcinoma cell proliferation, IC50=38 nM | 12190313 | |||
| ABAE human fibroblast cell | Proliferation assay | Inhibition of ABAE human fibroblast cell proliferation, IC50=45 nM | 12190313 | |||
| HL60 human leukemia cell | Proliferation assay | Inhibition of HL60 human leukemia cell proliferation, IC50=46 nM | 12190313 | |||
| Hs 27 human fibroblast cell | Proliferation assay | Inhibition of Hs 27 human fibroblast cell proliferation, IC50=51 nM | 12190313 | |||
| CCRF-CEM human leukemia cell | Proliferation assay | Inhibition of CCRF-CEM human leukemia cell proliferation, IC50=52 nM | 12190313 | |||
| OVCAR-3 human ovarian carcinoma cell | Proliferation assay | Inhibition of OVCAR-3 human ovarian carcinoma cell proliferation, IC50=54 nM | 12190313 | |||
| A2780/DDP-S human ovarian carcinoma cell | Proliferation assay | Inhibition of A2780/DDP-S human ovarian carcinoma cell proliferation, IC50=56 nM | 12190313 | |||
| A2780/TAX-S human ovarian carcinoma cell | Proliferation assay | Inhibition of A2780/TAX-S human ovarian carcinoma cell proliferation, IC50=65 nM | 12190313 | |||
| LS174T human colon carcinoma cell | Proliferation assay | Inhibition of LS174T human colon carcinoma cell proliferation, IC50=65 nM | 12190313 | |||
| MCF-7 human breast carcinoma cell | Proliferation assay | Inhibition of MCF-7 human breast carcinoma cell proliferation, IC50=66 nM | 12190313 | |||
| PC3 human prostate carcinoma cell | Proliferation assay | Inhibition of PC3 human prostate carcinoma cell proliferation, IC50=66 nM | 12190313 | |||
| MLF mouse lung fibroblast cell | Proliferation assay | Inhibition of MLF mouse lung fibroblast cell proliferation, IC50=72 nM | 12190313 | |||
| LX-1 human lung carcinoma | Proliferation assay | Inhibition of LX-1 human lung carcinoma proliferation, IC50=75 nM | 12190313 | |||
| A431 human squamous cell | Proliferation assay | Inhibition of A431 human squamous cell carcinoma cell proliferation, IC50=75 nM | 12190313 | |||
| SKBR-3 human breast carcinoma cell | Proliferation assay | Inhibition of SKBR-3 human breast carcinoma cell proliferation, IC50=77 nM | 12190313 | |||
| A2780/TAX-R human ovarian carcinoma cell | Proliferation assay | Inhibition of A2780/TAX-R human ovarian carcinoma cell proliferation, IC50=78 nM | 12190313 | |||
| M109 mouse lung carcinoma cell | Proliferation assay | Inhibition of M109 mouse lung carcinoma cell proliferation, IC50=80 nM | 12190313 | |||
| CACO-2 human colon carcinoma cell | Proliferation assay | Inhibition of CACO-2 human colon carcinoma cell proliferation, IC50=86 nM | 12190313 | |||
| A549 human lung carcinoma cell | Proliferation assay | Inhibition of A549 human lung carcinoma cell proliferation, IC50=96 nM | 12190313 | |||
| MIP human colon carcinoma cell | Function assay | Inhibition of MIP human colon carcinoma cell line, IC50=0.12 μM | 12190313 | |||
| K562 human leukemia cell | Proliferation assay | Inhibition of K562 human leukemia cell proliferation, IC50=0.13 μM | 12190313 | |||
| human A2780 cell line | Proliferation assay | 72 h | Antiproliferative effect against human A2780 cell line was determined in a whole cell 72 hr cytotoxicity assay, IC50=71 nM | 15027863 | ||
| human ovarian (A2780) cancer cell | Cytotoxic assay | Cytotoxic effect on human ovarian (A2780) cancer cell line, IC50=71 nM | 15125971 | |||
| ID8 cells | Proliferation assay | Antiproliferative activity against ID8 cells, IC50=7 nM | 17123821 | |||
| MCF7 cells | Proliferation assay | Antiproliferative activity against MCF7 cells, IC50=26 nM | 17123821 | |||
| Sf9 cells | Function assay | Inhibition of recombinant cyclin A/CDK2 expressed in Sf9 cells, IC50=12 nM | 17904366 | |||
| human A2780 cells | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 18469809 | |||
| human A2780 cells | Function assay | 24 h | Inhibition of cdk-mediated Rb phosphorylation at thr821 in human A2780 cells after 24 hrs | 18469809 | ||
| human NCI60 cells | Proliferation assay | 72 h | Antiproliferative activity against human NCI60 cells after 72 hrs by sulforhodamine B assay, GI50=74.7 nM | 21080703 | ||
| human NCI60 cells | Proliferation assay | 72 h | Antiproliferative activity against human NCI60 cells assessed as lethal effect after 72 hrs by sulforhodamine B assay, LC50=0.904 μM | 21080703 | ||
| human A2780 cells | Cytotoxic assay | 24 h | Cytotoxicity against human A2780 cells after 24 hrs by MTT assay, GI50=23 nM | 23301767 | ||
| human MRC5 cells | Cytotoxic assay | 72 h | Cytotoxicity against human MRC5 cells after 72 hrs by MTT assay, GI50=28 nM | 23301767 | ||
| human A2780 cells | Cytotoxic assay | 72 h | Cytotoxicity against human A2780 cells after 72 hrs by MTT assay, GI50=29 nM | 23301767 | ||
| human A2780 cells | Cytotoxic assay | 48 h | Cytotoxicity against human A2780 cells after 48 hrs by MTT assay, GI50=31 nM | 23301767 | ||
| human MRC5 cells | Cytotoxic assay | 48 h | Cytotoxicity against human MRC5 cells after 48 hrs by MTT assay, GI50=39 nM | 23301767 | ||
| human MRC5 cells | Cytotoxic assay | 24 h | Cytotoxicity against human MRC5 cells after 24 hrs by MTT assay, GI50=49 nM | 23301767 | ||
| human HMEC1 cells | Cytotoxic assay | 24 h | Cytotoxicity against human HMEC1 cells after 24 hrs by MTT assay, GI50=61 nM | 23301767 | ||
| human HMEC1 cells | Cytotoxic assay | 48 h | Cytotoxicity against human HMEC1 cells after 48 hrs by MTT assay, GI50=62 nM | 23301767 | ||
| human HMEC1 cells | Cytotoxic assay | 72 h | Cytotoxicity against human HMEC1 cells after 72 hrs by MTT assay, GI50=66 nM | 23301767 | ||
| A2780 | Function assay | 330 nM | 24 hr | Percentage A2780 cells in sb-G1 after 24 hr r at 330 nM (IC50), Cell cycle = 0.001 μM. | 12190313 | |
| A2780 | Apoptosis assay | 330 nM | 24 hr | Apoptotic A2780 cells after 24 hr r at 330 nM (IC50), Cell cycle = 0.001 μM. | 12190313 | |
| A2780 | Function assay | 2980 nM | 24 hr | Percentage A2780 cells in sub-G1 after 24 hr r at 2980 nM (IC90), Cell cycle = 0.002 μM. | 12190313 | |
| A2780 | Apoptosis assay | 2980 nM | 24 hr | Apoptotic A2780 cells after 24 hr r at 2980 nM (IC90), Cell cycle = 0.009 μM. | 12190313 | |
| A2780 | Function assay | 2980 nM | 24 hr | Percentage A2780 cells in S-phase after 24 hr r at 2980 nM (IC90), Cell cycle = 0.012 μM. | 12190313 | |
| A2780 | Function assay | 330 nM | 24 hr | Percentage A2780 cells in S-phase after 24 hr r at 330 nM (IC50), Cell cycle = 0.016 μM. | 12190313 | |
| A2780 | Function assay | 330 nM | 24 hr | Percentage A2780 cells in G2/M after 24 hr r at 330 nM (IC50), Cell cycle = 0.023 μM. | 12190313 | |
| A2780 | Function assay | 2980 nM | 24 hr | Percentage A2780 cells in G2/M after 24 hr r at 2980 nM (IC90), Cell cycle = 0.025 μM. | 12190313 | |
| A2780 | Function assay | 330 nM | 24 hr | Percentage A2780 cells in G1 after 24 hr r at 330 nM (IC50), Cell cycle = 0.06 μM. | 12190313 | |
| A2780 | Function assay | 2980 nM | 24 hr | Percentage A2780 cells in G1 after 24 hr r at 2980 nM (IC90), Cell cycle = 0.061 μM. | 12190313 | |
| A2780 | Function assay | 24 hrs | Inhibition of cdk-mediated Rb phosphorylation at ser807/811 in human A2780 cells after 24 hrs | 18469809 | ||
| A2780 | Function assay | 24 hrs | Inhibition of cdk9-mediated RNA pol2 CTD phosphorylation at ser2 in human A2780 cells after 24 hrs | 18469809 | ||
| A2780 | Function assay | 24 hrs | Inhibition of cdk7-mediated RNA pol2 CTD phosphorylation at ser5 in human A2780 cells after 24 hrs | 18469809 | ||
| A2780 | Apoptosis assay | 24 hrs | Induction of apoptosis in human A2780 cells assessed as appearance of Mcl1 protein level after 24 hrs | 18469809 | ||
| DR-U2OS-GFP | Function assay | 0.1 uM | 56 hrs | Reduction of homologous recombination in human DR-U2OS-GFP cells expressing I-SceI nuclease assessed as reduction of RAD51 level at 0.1 uM after 56 hrs by immunoblotting | 21417417 | |
| A2780 | Function assay | 24 hrs | Inhibition of CDK9 in human A2780 cells assessed as reduction of RNAPII CTD phosphorylation at Ser2 at GI50 concentration after 24 hrs by Western blotting analysis | 23301767 | ||
| A2780 | Cell cycle assay | 24 hrs | Cell cycle arrest in human A2780 cells assessed as accumulation at G2/M phase at less than GI50 after 24 hrs by flow cytometric analysis | 23301767 | ||
| A2780 | Function assay | 24 hrs | Inhibition of CDK9 in human A2780 cells assessed as downregulation of MCL1 at GI50 to 5XGI50 concentration after 24 hrs by Western blotting analysis | 23301767 | ||
| A2780 | Apoptosis assay | 24 hrs | Induction of apoptosis in human A2780 cells assessed as induction of PARP cleavage at GI50 to 5XGI50 concentration after 24 hrs by Western blotting analysis | 23301767 | ||
| A2780 | Function assay | 24 hrs | Inhibition of CDK9 in human A2780 cells assessed as downregulation of HDM2 at GI50 to 5XGI50 concentration after 24 hrs by Western blotting analysis | 23301767 | ||
| MT4 | Antiproliferative assay | Antiviral activity against Human immunodeficiency virus 1 NL 4-3 infected in MT4 cells measured on day 4 post infection by p24 assay, EC50 = 0.015 μM. | 25914804 | |||
| MT4 | Cytotoxicity assay | Cytotoxicity against human MT4 cells, IC50 = 0.067 μM. | 25914804 | |||
| Sf9 | Function assay | 15 mins | Inhibition of CDK2/cyclin E1 (unknown origin) expressed in Sf9 insect cells using UlightCFFKNIVTPRTPPPSQGK-amide substrate after 15 mins by autoradiography, IC50 = 0.13 μM. | 25914804 | ||
| A549 | Antiproliferative assay | 3 days | Antiproliferative activity against human A549 cells after 3 days by SRB method, GI50 = 0.14 μM. | 25914804 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against human DU145 cells after 3 days by SRB method, GI50 = 0.15 μM. | 25914804 | ||
| KB | Antiproliferative assay | 3 days | Antiproliferative activity against human KB cells after 3 days by SRB method, GI50 = 0.16 μM. | 25914804 | ||
| KBVIN | Antiproliferative assay | 3 days | Antiproliferative activity against human KBVIN cells after 3 days by SRB method, GI50 = 0.18 μM. | 25914804 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by Celltiter-Glo reagent based assay in presence of 10% fetal bovine serum, EC50 = 0.034 μM. | 26985305 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by Celltiter-Glo reagent based assay in presence of 0.625% fetal bovine serum, EC50 = 0.059 μM. | 26985305 | ||
| Sf9 | Function assay | 10 mins | Inhibition of human His6-tagged CDK9/cyclin T1 expressed in baculovirus infected sf9 cells using GST-CTD as substrate after 10 mins in presence of [gamma-32P]ATP by SDS-PAGE analysis, IC50 = 0.0025 μM. | 27171036 | ||
| Sf21 | Function assay | Inhibition of recombinant human full length C-terminal His6-tagged CDK9/cyclin T1 expressed in baculovirus infected Sf21 insect cells using PDKtide as substrate, IC50 = 0.011 μM. | 27171036 | |||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as decrease in cell viability after 72 hrs by MTT assay, CC50 = 0.12 μM. | 27171036 | ||
| sf21 | Function assay | Inhibition of full length human N-terminal His6-tagged CDK6/N-terminal GST-tagged cyclin D3 expressed in sf21 cells using histone H1 substrate, IC50 = 0.395 μM. | 27171036 | |||
| Sf21 | Function assay | Inhibition of recombinant human full length C-terminal His6-tagged CDK7/cyclin H/N-terminal GST-tagged MAT1 expressed in baculovirus infected Sf21 insect cells using cdk7 substrate peptide, IC50 = 0.514 μM. | 27171036 | |||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.1464 μM. | 29407975 | ||
| KOPN8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KOPN8 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.1926 μM. | 29407975 | ||
| SEM | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SEM cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.2043 μM. | 29407975 | ||
| UOCB1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human UOCB1 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.2084 μM. | 29407975 | ||
| KOPN8 | Apoptosis assay | 0.5 uM | 3 to 24 hrs | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM after 3 to 24 hrs by Western blot analysis | 29407975 | |
| KOPN8 | Apoptosis assay | 0.5 uM | 1 hr | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM pre-treated with NAC for 1 hr and measured after 3 to 24 hrs by Western blot analysis | 29407975 | |
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 401.84 | Formula | C21H20ClNO5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 146426-40-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 649890, HMR-1275, L86-8275 | Smiles | CN1CCC(C(C1)O)C2=C(C=C(C3=C2OC(=CC3=O)C4=CC=CC=C4Cl)O)O | ||
Solubility
|
In vitro |
DMSO
: 80 mg/mL
(199.08 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
First CDK inhibitor to be used in human clinical trials.
|
|---|---|
| Targets/IC50/Ki | |
| In vitro |
Flavopiridol (Alvocidib) displays less activity against unrelated kinases such as MAP, PAK, PKC, and EGFR with IC50 of >14 μM. It significantly inhibits the colony growth of HCT116, A2780, PC3, and Mia PaCa-2 cells with IC50 of 13 nM, 15 nM, 10 nM and 36 nM, respecitively. [1] This compound also potently inhibits the activity of Glycogen synthase kinase-3 (GSK-3) with an IC50 of 280 nm. [2] Compared with other CDKs, it inhibits the activity of CDK7 less potently with IC50 of 875 nM. At 0.5 μM, it inhibits both pSer807/811 Rb and pThr199 NPM, whereas mild changes are observed at pThr821 Rb. It also decreases the overall RNA polymerase II level, as well as the phosphorylation of RNA polymerase II on the CTD repeats at Ser2 Ser5. [3] As a broad spectrum CDK inhibitor, it can inhibit cell cycle progression in either G1 or G2. At 0.3 μM, it induces G1 arrest in either MCF-7 or MDA-MB-468 cells by inhibition of the CDK4 or CDK2 kinase activity. [4] It exhibits potent cytotoxicity against a wide variety of tumor cell lines with IC50 values ranging form 16 nM for LNCAP to 130 nM for K562. [5]
|
| Kinase Assay |
CDK kinase assay
|
|
For CDK1/cyclin B1 kinase assay, kinase reactions consist of 100 ng of baculovirus expressed GST-CDK1/cyclin B1 (human) complex, 1 μg histone HI, 0.2 μCi [γ-33P]ATP, 25 μM ATP in 50 μL kinase buffer (50 mM Tris, pH 8.0, 10 mM MgCl2, 1 mM EGTA, 0.5 mM DTT). For CDK2/cyclin E kinase assay, kinase reactions consist of 5 ng of baculovirus expressed GST-CDK2/cyclin E (human) complex, 0.5 μg GST-RB fusion protein (amino acids 776-928 of retinoblastoma protein), 0.2 μCi [γ-33P]ATP, 25 μM ATP in 50 μL kinase buffer (50 mM Hepes, pH 8.0, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT). For CDK4/cyclin D1 kinase assay, kinase reactions consist of 150 ng of baculovirus expressed GST-CDK4/cyclin D1 (human), 280 ng of Stag-cyclin D1, 0.5 μg GST-RB fusion protein (amino acids 776-928 of retinoblastoma protein), 0.2 μCi [γ-33P]ATP, 25 μM ATP in 50 μL kinase buffer (50 mM Hepes, pH 8.0, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT). Reactions are incubated for 45 minutes for CDK1 and CDK2, or 1 hour for CDK4 at 30 °C and stopped by the addition of cold trichloroacetic acid (TCA) to a final concentration 15%. TCA precipitates are collected onto GF/C unifilter plates using a Filtermate universal harvester and the filters are quantitated using a TopCount 96-well liquid scintillation counter. Flavopiridol (Alvocidib) is dissolved at 10 mM in dimethylformamide (DMF) and evaluated at six concentrations, each in triplicate. The final concentration of DMF in the assay = 2%. IC50 values are derived by nonlinear regression analysis and have a coefficient of variance = 16%. To assay its activity on CDK6, a filter-binding assay is established. The following are combined in the reaction mixture: 2 μL of CDK6 (0.7 mg/μL), 5 μL of histone H1 (6 mg/mL), 14 μL of kinase buffer (60 mM β-glycerophosphate, 30 mM p-nitrophenyl phosphate, 25 mM MOPS (pH 7.0), 5 mM EGTA, 15 mM MgCl2, 1 mM DTT, 0.1 mM Na-vanadate), 3 μL of increasing concentrations of this compound diluted in 50% DMSO, and 6 μL of 33P-ATP (1 mCi/mL) in nonradioactive ATP at 90 μM concentration (final concentration: 15 μM). The assay is initiated by the addition of 33P-ATP. The reaction is incubated for 20 minutes at 30°C. A 25 μL aliquot of the supernatant is then spotted onto Whatman P81 phosphocellulose paper. Filters are washed 5 times with 1% phosphoric acid solution. Wet filters are counted in the presence of 1 mL of scintillation fluid. Cdk9 activity is measured using 50 nM of recombinant Cdk9/cyclin T in 50 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT, 3 μM Na3VO4, 150 μM RNA polymerase CDT peptide and 80 μM ATP. Cdk7 assay is performed in the same buffer using 37 nM of purified kinase in the presence of 200 μM ATP and 10 μM myelin binding protein as a substrate. The potency of it toward CDK9 and CDK7 is determined using either a strong anion exchanger (Dowex 1-X8 resin, formate form)-based assay or a scintillation proximity assay. IC50 values are calculated from the dose-response curves.
|
|
| In vivo |
Administration of S1230 at 7.5 mg/kg for 7 days displays slight antitumor activity against P388 murine leukemia, resulting in %T/C value of 110, and active against the human A2780 ovarian carcinoma implanted sc in nude mice, producing 1.5 log cell kill (LCK). [5] S1230 treatment at 1-2.5 mg/kg for 10 days significantly suppresses collagen-induced arthritis in mice in a dose-dependent manner, by inhibiting synovial hyperplasia and joint destruction, whereas serum concentrations of anti-collagen type II (CII) Abs and proliferative responses to CII are maintained. [6] In the p21-intact Hct116 xenografts in nude mice, administration of CPT-11 (100 mg/kg) followed by S1230 (3 mg/kg) 7 and 16 hours later significantly inhibits tumor regression by 86% and 82%, respectively, displaying >2 fold inhibition compared with CPT-11 alone by 40 %. The combination produces ~30% complete response rate (CR) in contrast to CPT-11 alone where no CR is found. [7]
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | Cleaved caspase-8 / Cleaved caspase-9 / Cleaved caspase-3 p-RNAPII / p-eIF4E / Mnk1 p-ERK / ERK / p-p38 / p-4EBP1 / 4EBP1 / p-S6 CDK2 / CDK4 / Cyclin A / p21 / p27 / Rb |
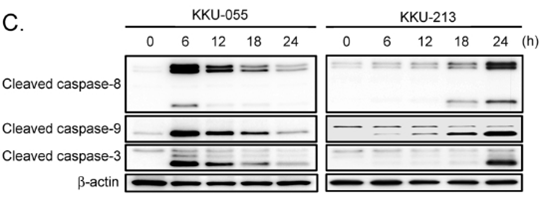
|
31193061 |
| Growth inhibition assay | Cell viability |
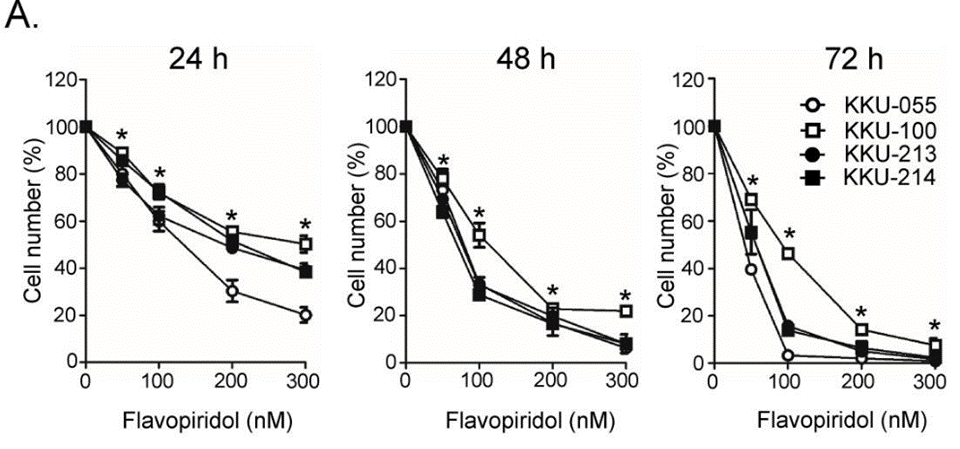
|
31193061 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03441555 | Completed | Acute Myeloid Leukemia (AML) |
AbbVie|Sumitomo Pharma America Inc. |
May 30 2018 | Phase 1 |
| NCT03298984 | Completed | Acute Myeloid Leukemia |
Sumitomo Pharma America Inc. |
September 25 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































