research use only
Riviciclib hydrochloride (P276-00) CDK inhibitor
Cat.No.S8058
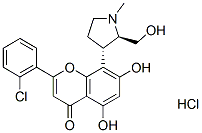
Chemical Structure
Molecular Weight: 438.3
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| KB-8-5-11 | qHTS assay | P-glycoprotein substrates identified in KB-8-5-11 adenocarcinoma cell line, qHTS therapeutic library screen, Potency = 5.1735 μM. | 31515284 | |||
| KB-3-1 | qHTS assay | P-glycoprotein substrates identified in KB-3-1 adenocarcinoma cell line, qHTS therapeutic library screen. | 31515284 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 88 mg/mL
(200.77 mM)
Water : 88 mg/mL Ethanol : 7 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 438.3 | Formula | C21H20ClNO5.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 920113-03-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CN1CCC(C1CO)C2=C(C=C(C3=C2OC(=CC3=O)C4=CC=CC=C4Cl)O)O.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
CDK9/CyclinT1
20 nM
CDK4/CyclinD1
63 nM
CDK1/CyclinB
79 nM
CDK2/CyclinA
224 nM
CDK6/CyclinD3
396 nM
CDK2/CyclinE
2.54 μM
GSK-3β
2.771 μM
CDK7/CyclinH
2.87 μM
|
|---|---|
| In vitro |
P276-00 shows 40-fold selectivity toward Cdk4-D1, compared with Cdk2-E. It shows potent antiproliferative effects against various human cancer cell lines, including HCT-116, U2OS, H-460, HL-60, HT-29, SiHa, MCF-7, Colo-205, SW-480, PC-3, Caco2, T-24 with an IC50 ranging from 300 to 800 nmol/L, and is found to be highly selective for cancer cells as compared with normal fibroblast cells. This compound can down-regulate cyclin D1 and Cdk4 in an ATP- competitive manner and decrease Cdk4-specific pRb Ser780 phosphorylation. It also induces apoptosis by actving cellular caspase-3 activity and DNA ladder formation.
|
| Kinase Assay |
Cdk4-D1/Cdk2-E enzyme assay
|
|
The Cdk4-D1/Cdk2-E enzyme assay is run in 96-well format using Millipore Multiscreen filtration plates. All assay steps are done in a single filter plate. The filtration wells are prewetted with 100 μL of kinase buffer [50 mmol/L HEPES (pH, 7.5), 10 mmol/L MgCl2, 1 mmol/L EGTA], and then the solution is removed by vacuum. With filter plate on vacuum manifold, 50 μL GST-Rb bound to GSH-Sepharose beads in kinase buffer (0.5 μg GST-Rb/50 μL) is added to each well, and vacuum is applied to the filter plate. About 25 μL of a reaction mix containing ATP (cold + hot) and 4× phosphatase inhibitor mix (40 μmol/L unlabeled ATP, 10 μCi/mL γ32P-ATP, 40 mmol/L h-glycerophosphate, 4 mmol/L DTT, 0.4 mmol/L NaF, 0.4 mmol/L sodium orthovanadate)
diluted in kinase buffer is added to each well. The test compound (4×final concentration in kinase buffer) or kinase buffer alone (control) is then added in an additional 25 μL volume. To each well, 50 μL (100 ng) of human Cdk4-D1/Cdk2-E enzyme in kinase buffer is added to initiate the reaction, which is allowed to continue for 30 min at 30°C.
When the reaction is completed, vacuum is applied again, and the plate is washed with the TNEN buffer [20 mmol/L Tris (pH, 8.0), 100 mmol/L NaCl, 1 mmol/L EDTA, 0.5% nonidet-P40] thrice; the filter plate is air-dried and is placed in a Multiscreen adapter plate. Packard Microscint-O cocktail (30 μL) is added, and the plate is covered with a Top-Seal A film. Quantitation of 32P-GST-Rb in 96-well filter plates is carried out by Top Count scintillation counter. All compounds are tested initially at 1 μmol/L concentration. Compounds showing more than or equal to 50% inhibition are further profiled for IC50 determination.
|
|
| In vivo |
P276-00, administered i.p. at 50 mg/kg daily for 20 treatments can significantly induce growth inhibition of murine colon cancer (CA-51). However, in murine lung carcinoma model (Lewis lung), an increased dose of 60 mg/kg (30 mg/kg twice daily) administered every alternate day i.p. for 7 treatments shows significant inhibition in the growth. And this compound also inhibit the growth of human colon carcinoma HCT-116 xenograft and human non-small cell lung carcinoma H-460 xenograft. Efficacy Studies show its maximum tolerated dose is 78 mg/kg/d.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00899054 | Completed | Squamous Cell Carcinoma of Head and Neck |
Piramal Enterprises Limited |
August 2009 | Phase 1|Phase 2 |
| NCT00898287 | Completed | Pancreatic Cancer |
Piramal Enterprises Limited |
May 2009 | Phase 1|Phase 2 |
| NCT00547404 | Withdrawn | Multiple Myeloma |
Piramal Enterprises Limited |
December 2008 | Phase 1 |
| NCT00882063 | Completed | Relapsed and/or Refractory Multiple Myeloma |
Piramal Enterprises Limited |
January 2008 | Phase 1|Phase 2 |
| NCT00407498 | Completed | Neoplasm |
Piramal Enterprises Limited |
May 2005 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































