research use only
Clofarabine DNA/RNA Synthesis inhibitor
Cat.No.S1218
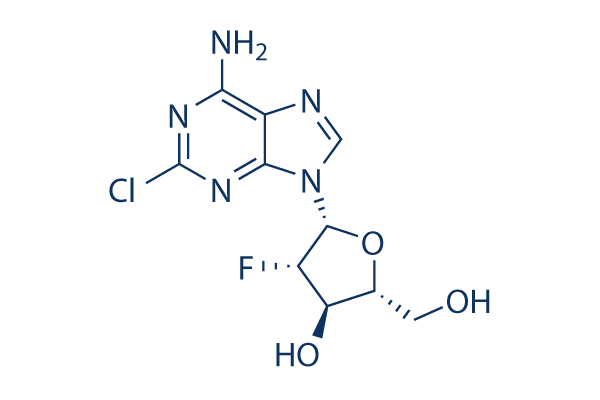
Chemical Structure
Molecular Weight: 303.68
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| K562 cell | Cytotoxicity assay | Compound was tested for cytotoxicity against K562 cell lines, IC50=0.003 μM | 1732556 | |||
| HEp-2 cell | Cytotoxicity assay | Compound was tested for cytotoxicity against HEp-2 cell lines, IC50=0.012 μM | 1732556 | |||
| CCRF-CEM cell lines | Cytotoxicity assay | Compound was tested for cytotoxicity against CCRF-CEM cell lines, IC50=0.05 μM | 1732556 | |||
| L1210 cell | Cytotoxicity assay | Compound was tested for cytotoxicity against L1210 cell lines, IC50=2.3 μM | 1732556 | |||
| NCI-H23 | Cytotoxicity assay | 5 days | Cytotoxicity against human NCI-H23 cells after 5 days by SRB assay, GI50=0.04μM | 19929004 | ||
| PC3 | Cytotoxicity assay | 5 days | Cytotoxicity against human PC3 cells after 5 days by SRB assay, GI50=0.063μM | 19929004 | ||
| BT549 | Cytotoxicity assay | 5 days | Cytotoxicity against human BT549 cells after 5 days by SRB assay, GI50=0.065μM | 19929004 | ||
| HCT15 | Cytotoxicity assay | 5 days | Cytotoxicity against human HCT15 cells after 5 days by SRB assay, GI50=0.18μM | 19929004 | ||
| NCI-H23 | Cytostatic assay | 5 days | Cytostatic activity against human NCI-H23 cells after 5 days by SRB assay, GI50=0.04μM | 21711054 | ||
| MT4 | Cytostatic assay | 5 days | Cytostatic activity against human MT4 cells after 5 days by SRB assay, GI50=0.051μM | 21711054 | ||
| PC3 | Cytostatic assay | 5 days | Cytostatic activity against human PC3 cells after 5 days by SRB assay, GI50=0.063μM | 21711054 | ||
| BT549 | Cytostatic assay | 5 days | Cytostatic activity against human BT549 cells after 5 days by SRB assay, GI50=0.065μM | 21711054 | ||
| A549 | Cytostatic assay | 5 days | Cytostatic activity against human A549 cells after 5 days by SRB assay, GI50=0.086μM | 21711054 | ||
| HCT116 | Cytostatic assay | 5 days | Cytostatic activity against human HCT116 cells after 5 days by SRB assay, GI50=0.106μM | 21711054 | ||
| DU145 | Cytostatic assay | 5 days | Cytostatic activity against human DU145 cells after 5 days by SRB assay, GI50=0.125μM | 21711054 | ||
| HCT15 | Cytostatic assay | 5 days | Cytostatic activity against human HCT15 cells after 5 days by SRB assay, GI50=0.18μM | 21711054 | ||
| Hs578 | Cytostatic assay | 5 days | Cytostatic activity against human Hs578 cells after 5 days by SRB assay, GI50=1.241μM | 21711054 | ||
| HL60 | Cytostatic assay | 48 hrs | Cytostatic activity against human HL60 cells after 48 hrs by MTT assay, IC50=0.1μM | 23820572 | ||
| A549 | Cytostatic assay | 48 hrs | Cytostatic activity against human A549 cells after 48 hrs by MTT assay, IC50=8μM | 23820572 | ||
| U373-MAGI | Function assay | 50 nM | 2 hrs | Potentiation of 5-Aza-C-induced antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as 5-Aza-C EC50 at 50 nM preincubated for 2 hrs followed by 5-Aza-C addition for 2 hrs and subsequent viral infection measu, EC50=30.4μM | 27117260 | |
| U373-MAGI | Antiviral assay | 50 nM | 4 hrs | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in viral infectivity at 50 nM incubated for 4 hrs prior to viral infection measured at 72 hrs post infection by flow cytometric analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 6 hrs | Reduction in dGTP level in human U373-MAGI cells at 200 nM after 6 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 6 hrs | Reduction in dCTP level in human U373-MAGI cells at 200 nM after 6 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 6 hrs | Reduction in dATP level in human U373-MAGI cells at 200 nM after 6 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 50 nM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 50 nM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 200 nM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-C | 27117260 | |
| U373-MAGI | Function assay | 50 nM | 2 hrs | Increase in 5-aza-dCTP/dCTP ratio in human U373-MAGI cells at 50 nM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 2 hrs | Increase in 5-aza-dCTP/dCTP ratio in human U373-MAGI cells at 200 nM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 2 hrs | Reduction in dRGU-TP level in human U373-MAGI cells at 200 nM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 2 hrs | Reduction in 5-aza-dCTP level in human U373-MAGI cells at 200 nM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 200 nM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 200 nM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 50 nM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 50 nM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for SJ-GBM2 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for MG 63 (6-TG R) cells | 29435139 | |||
| Granta | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Granta cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50=0.017μM | 30176535 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50=0.04μM | 30176535 | ||
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50=0.044μM | 30176535 | ||
| RL | Cytotoxicity assay | 72 hrs | Cytotoxicity against human RL cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50=0.38μM | 30176535 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 60 mg/mL
(197.57 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 303.68 | Formula | C10H11ClFN5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 123318-82-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Clolar | Smiles | C1=NC2=C(N=C(N=C2N1C3C(C(C(O3)CO)O)F)Cl)N | ||
Mechanism of Action
| Targets/IC50/Ki |
Ribonucleotide reductase
(Cell-free assay) 65 nM
|
|---|---|
| In vitro |
Clofarabine is efficiently transported into cells via two facilitative or equilibrative nucleoside transporters, hENT1 and hENT2, and a concentrative nucleoside transporter, hCNT253. This compound is phosphorylated in a stepwise manner by cytosolic kinases to the nucleotide analogues clofarabine 5′-mono-, di- and triphosphate following entry into cells, with Clofarabine triphosphate being the active form. Clofarabine 5′-mono-, di- and triphosphate are not substrates for nucleoside transporters and must be enzymatically converted by 5′-nucleotidase back to their dephosphorylated nucleoside form for transport out of the cell. This compound triphosphate is a potent inhibitor of ribonucleotide reductase (IC50 = 65 nM), presumably by binding to the allosteric site on the regulatory subunit. It has also been shown to act directly on mitochondria by altering the transmembrane potential with release of cytochrome c, apoptotic-inducing factor (AIF), apoptosis protease-activating factor 1 (APAF1) and caspase 9 into the cytosol. This chemical demonstrates strong in vitro growth inhibition and cytotoxic activity (IC50 values = 0.028–0.29 μM) in a wide variety of leukaemia and solid tumour cell lines. It has been shown to increase the activity of dCK in HL60 cells, and increases the formation of the mono-, di-, and triphosphates of ara-C in K562 cells36. This compound (10 μM) inhibits the repair initiated by 4-hydroperoxycyclophosphamide (4-HC), with inhibition peaking at the intracellular concentrations of 5 μM in chronic lymphocytic leukemia (CLL) lymphocytes. It (10 μM) combined with 4-hydroperoxycyclophosphamide (4-HC) produces more than additive apoptotic cell death than the sum of each alone. This chemical (1 μM) combined with ara-C (10 μM) results in a biochemical modulation of ara-CTP and synergistic cell kill in K562 cells.
|
| In vivo |
Clofarabine administered intraperitoneally has significant activity against a wide variety of human tumour xenografts implanted subcutaneously in athymic nude or severe combined immune deficiency mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05917405 | Recruiting | Acute Myeloid Leukemia in Remission |
Nantes University Hospital |
September 14 2023 | Phase 2 |
| NCT03609814 | Completed | Hematologic Malignancies|Nonmalignant Diseases|Immunodeficiencies|Hemoglobinopathies|Genetic Inborn Errors of Metabolism|Fanconi''s Anemia|Thalassemia|Sickle Cell Disease |
University of California San Francisco |
January 26 2016 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































