research use only
Triapine (3-AP) DNA/RNA Synthesis inhibitor
Cat.No.S7470
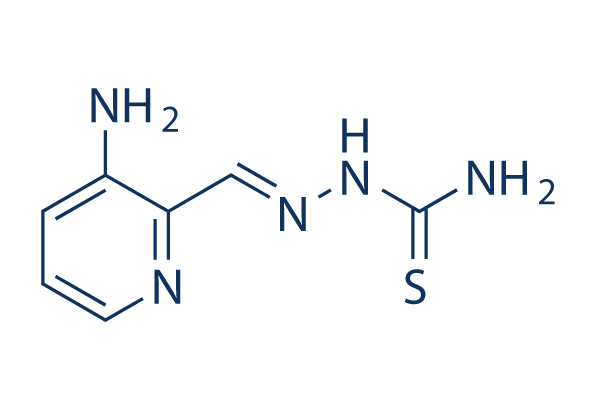
Chemical Structure
Molecular Weight: 195.24
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Carmofur YK-4-279 Halofuginone |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.31μM | 17142046 | |||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 17602603 | ||
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.54μM | 17963372 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 18159922 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.4μM | 19397322 | ||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.41254μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.88844μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.9μM | 19397322 | ||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 19601577 | ||
| HCT116 | Dark cytotoxicity assay | 96 hrs | Dark cytotoxicity against human HCT116 cells expressing wild type p53 after 96 hrs by MTT assay, IC50=1.226μM | 24900837 | ||
| HCT116 | Photocytotoxicity assay | 24 hrs | Photocytotoxicity against human HCT116 cells expressing wild type p53 incubated for 24 hrs followed by light irradiation measured after 24 hrs by MTS assay in presence of ALA and FeCl3 | 24900837 | ||
| HCT116 | Function assay | 4 uM | 24 hrs | Induction of ROS generation in human HCT116 cells expressing wild type p53 at 4 uM after 24 hrs by spectrophotometry | 24900837 | |
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 27336684 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 27336684 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB-3-1 cells incubated for 72 hrs by MTT assay, IC50=3.1μM | 27336684 | ||
| KBC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBC1 cells incubated for 72 hrs by MTT assay, IC50=8.9μM | 27336684 | ||
| SW480 | Function assay | 2.5 uM | 48 hrs | Induction of morphological changes in human SW480 cells assessed as induction of massive cell flattening at 2.5 uM incubated for 48 hrs by phase contrast microscopy | 27336684 | |
| HL60 | Function assay | 1 and 5 uM | 30 mins | Induction of intracellular ROS generation in human HL60 cells at 1 and 5 uM in presence of CuCl2 incubated for 30 mins by DCF-DA staining based fluorescence assay | 27336684 | |
| L1210 | Growth inhibition assay | Growth inhibition of mouse L1210 cells by MTS assay, IC50=1.3μM | 30904782 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 31614257 | |||
| WPMY-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WPMY-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=4.96μM | 31614257 | ||
| GES-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GES-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=5.6μM | 31614257 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells (CO-ADD:MA_007); CC50 by cell viability assay in DMEM (10% FBS) media using TC plates, by Resazurin F(560/590), CC50=2.827μM | ChEMBL | |||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells after 72 hrs by MTT assay, IC50=5.4μM | ChEMBL | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50=9.68μM | ChEMBL | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50=18.85μM | ChEMBL | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50=42.81μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 39 mg/mL
(199.75 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 195.24 | Formula | C7H9N5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 200933-27-3 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
Ribonucleotide reductase
|
|---|---|
| In vitro |
Triapine (3-AP) potently inhibits the activity of ribonucleotide reductase in both wild-type KB and HU-resistant KB nasopharyngeal carcinoma cells, and shows broad spectrum antitumor activity by inhibiting DNA synthesis in a series of cancer cell lines. In vitro, it blocks ischemic neurotoxicity and hypoxic toxicity with EC50 of 0.35 μM and 0.75 μM, respectively. This compound also shows its neuroprotective activity by suppressing cell death induced by neurotoxic agents, including staurosporine, veratridine and glutamate.
|
| Kinase Assay |
Ribonucleotide reductase assay
|
|
CDP reductase is assayed using Dowex 1-borate ion-exchange chromatography. The assay mixture contains 0.02 μCi of [14C]CDP (52.9 mCi/mmol), 3 mM dithiothreitol, 6 mM MgCl2, 30 mM HEPES, 5 mM ATP, 0.15 mM unlabeled CDP, and 10 μL of cellular extract in a final volume of 0.02 mL. The incubation time for the reaction is 60 min, during which time the reaction is linear.
|
|
| In vivo |
In mice bearing the L1210 leukemia, Triapine (3-AP) (1.25 to 20 mg/kg) is curative for some mice without lethal toxicity. This compound also inhibits the growth of solid tumors in mice M109 lung carcinoma and human A2780 ovarian carcinoma xenografts. In addition, its combination with various classes of agents that damage DNA results in synergistic inhibition of the L1210 leukemia. In a rat model of transient ischemia, it reduces infarct volume by 59% when administered i.c.v. (50 μ per rat) and by 35% when administered i.v. (1 mg/kg).
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-Chk1 / Chk1 / p-CDK1/2 / CDK1/2 / p-H1 |
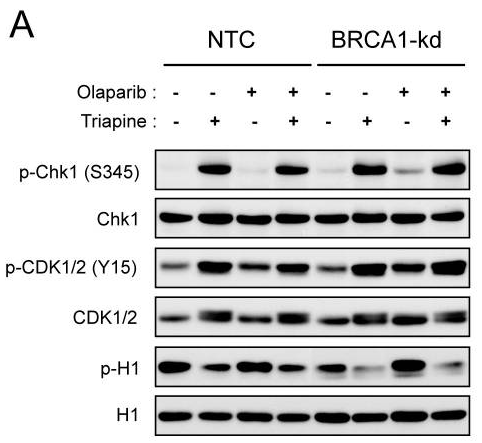
|
24413181 |
| Growth inhibition assay | Cell viability |
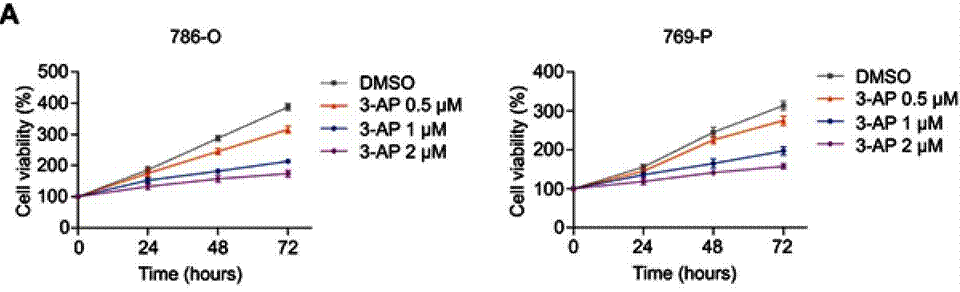
|
31118677 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00293345 | Completed | Anaplastic Large Cell Lymphoma|Angioimmunoblastic T-cell Lymphoma|Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Intraocular Lymphoma|Nodal Marginal Zone B-cell Lymphoma|Primary Central Nervous System Hodgkin Lymphoma|Primary Central Nervous System Non-Hodgkin Lymphoma|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Hodgkin Lymphoma|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Adult T-cell Leukemia/Lymphoma|Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|Recurrent Small Lymphocytic Lymphoma|Small Intestine Lymphoma|Splenic Marginal Zone Lymphoma|Stage III Adult Burkitt Lymphoma|Stage III Adult Diffuse Large Cell Lymphoma|Stage III Adult Diffuse Mixed Cell Lymphoma|Stage III Adult Diffuse Small Cleaved Cell Lymphoma|Stage III Adult Hodgkin Lymphoma|Stage III Adult Immunoblastic Large Cell Lymphoma|Stage III Adult Lymphoblastic Lymphoma|Stage III Adult T-cell Leukemia/Lymphoma|Stage III Cutaneous T-cell Non-Hodgkin Lymphoma|Stage III Grade 1 Follicular Lymphoma|Stage III Grade 2 Follicular Lymphoma|Stage III Grade 3 Follicular Lymphoma|Stage III Mantle Cell Lymphoma|Stage III Marginal Zone Lymphoma|Stage III Mycosis Fungoides/Sezary Syndrome|Stage III Small Lymphocytic Lymphoma|Stage IV Adult Burkitt Lymphoma|Stage IV Adult Diffuse Large Cell Lymphoma|Stage IV Adult Diffuse Mixed Cell Lymphoma|Stage IV Adult Diffuse Small Cleaved Cell Lymphoma|Stage IV Adult Hodgkin Lymphoma|Stage IV Adult Immunoblastic Large Cell Lymphoma|Stage IV Adult Lymphoblastic Lymphoma|Stage IV Adult T-cell Leukemia/Lymphoma|Stage IV Cutaneous T-cell Non-Hodgkin Lymphoma|Stage IV Grade 1 Follicular Lymphoma|Stage IV Grade 2 Follicular Lymphoma|Stage IV Grade 3 Follicular Lymphoma|Stage IV Mantle Cell Lymphoma|Stage IV Marginal Zone Lymphoma|Stage IV Mycosis Fungoides/Sezary Syndrome|Stage IV Small Lymphocytic Lymphoma|Unspecified Adult Solid Tumor Protocol Specific|Waldenström Macroglobulinemia |
National Cancer Institute (NCI) |
June 2006 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































