research use only
LEE011 (Ribociclib) CDK4/6 inhibitor
Cat.No.S7440
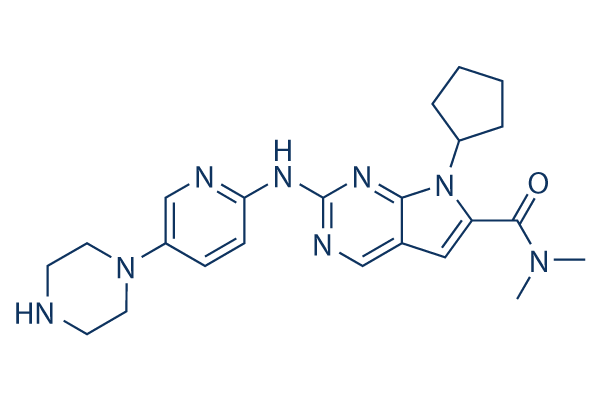
Chemical Structure
Molecular Weight: 434.54
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| DFSP105 | Growth Inhibition Assay | 24 h | GI50=276 nM | 25852058 | ||
| Myoblast | Growth Inhibition Assay | 72 h | IC50=1035 nM | 25810375 | ||
| IMRS | Growth Inhibition Assay | 72 h | IC50=873 nM | 25810375 | ||
| SKNAS | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| Rh28 | Growth Inhibition Assay | 72 h | IC50=845 nM | 25810375 | ||
| Rh41 | Growth Inhibition Assay | 72 h | IC50=7187 nM | 25810375 | ||
| CW9019 | Growth Inhibition Assay | 72 h | IC50=9912 nM | 25810375 | ||
| Rh5 | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| Rh30 | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| 778 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| 449 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP3 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP6 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP8 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LPS141 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| 778 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| 449 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP3 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP6 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP8 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LPS141 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| IMR5 | Growth Inhibition Assay | 24 h | DMSO | IC50=126 nM | 24045179 | |
| BE2C | Growth Inhibition Assay | 24 h | DMSO | IC50=134 nM | 24045179 | |
| 1643 | Growth Inhibition Assay | 24 h | DMSO | IC50=147 nM | 24045179 | |
| SKNSH | Growth Inhibition Assay | 24 h | DMSO | IC50=148 nM | 24045179 | |
| SY5Y | Growth Inhibition Assay | 24 h | DMSO | IC50=154 nM | 24045179 | |
| NGP | Growth Inhibition Assay | 24 h | DMSO | IC50=175 nM | 24045179 | |
| KELLY | Growth Inhibition Assay | 24 h | DMSO | IC50=220 nM | 24045179 | |
| CHP134 | Growth Inhibition Assay | 24 h | DMSO | IC50=273 nM | 24045179 | |
| NLF | Growth Inhibition Assay | 24 h | DMSO | IC50=328 nM | 24045179 | |
| LAN5 | Growth Inhibition Assay | 24 h | DMSO | IC50=429 nM | 24045179 | |
| NB69 | Growth Inhibition Assay | 24 h | DMSO | IC50=738 nM | 24045179 | |
| SKNDZ | Growth Inhibition Assay | 24 h | DMSO | IC50=801 nM | 24045179 | |
| NBSD | Growth Inhibition Assay | 24 h | DMSO | IC50=1900 nM | 24045179 | |
| SKNF1 | Growth Inhibition Assay | 24 h | DMSO | IC50=3500 nM | 24045179 | |
| EBC1 | Growth Inhibition Assay | 24 h | DMSO | IC50=6400 nM | 24045179 | |
| SKNAS | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| NB16 | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| RPE1 | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| Sf21 | Function assay | 10 mins | Inhibition of recombinant human full length N-terminal GST-tagged CDK4/Cyclin-D3 co-expressed in baculovirus infected sf21 cells using Rb substrate in presence of [gamma33P]ATP after 10 mins by scintillation counting method, IC50 = 0.013 μM. | 29518312 | ||
| Sf21 | Function assay | 10 mins | Inhibition of recombinant human full length C-terminal 6His-tagged CDK9/Cyclin-T1 co-expressed in baculovirus infected sf21 cells using PDKtide substrate in presence of [gamma33P]ATP after 10 mins by scintillation counting method, IC50 = 0.197 μM. | 29518312 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.2862 μM. | 29407975 | ||
| SEM | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SEM cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.4605 μM. | 29407975 | ||
| KOPN8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KOPN8 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.5008 μM. | 29407975 | ||
| NCI-H1299 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1299 cells assessed as reduction in cell viability after 72 hrs by CCK8 assay, IC50 = 5.46 μM. | 29518312 | ||
| T47D | Growth inhibition assay | 72 hrs | Growth inhibition of human T47D cells incubated for 72 hrs by CCK8 assay, IC50 = 6.227 μM. | 28651979 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells assessed as reduction in cell viability after 72 hrs by CCK8 assay, IC50 = 6.23 μM. | 29518312 | ||
| H1299 | Growth inhibition assay | 72 hrs | Growth inhibition of human H1299 cells incubated for 72 hrs by CCK8 assay, IC50 = 7.637 μM. | 28651979 | ||
| KOPN8 | Apoptosis assay | 0.5 uM | 3 hrs | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM after 3 hrs by Western blot analysis | 29407975 | |
| KOPN8 | Apoptosis assay | 0.5 uM | 24 hrs | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM pre-treated with NAC for 1 hr and measured after 24 hrs by Western blot analysis | 29407975 | |
| Hep3B | Cell cycle assay | 24 hrs | Cell cycle arrest in human Hep3B cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| HepG2 | Cell cycle assay | 24 hrs | Cell cycle arrest in human HepG2 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| A549 | Cell cycle assay | 24 hrs | Cell cycle arrest in human A549 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| NCI-H460 | Cell cycle assay | 24 hrs | Cell cycle arrest in human NCI-H460 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| T47D | Cell cycle assay | 24 hrs | Cell cycle arrest in human T47D cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| MDA-MB-231 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MDA-MB-231 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| Fluc-labeled 4T1 | Antitumor assay | 130 mg/kg | 18 days | Antitumor activity against mouse Fluc-labeled 4T1 cells implanted in Balb/c mouse assessed as reduction in tumor weight at 130 mg/kg, ip administered daily for 18 days measured after 8 to 25 days | 29518312 | |
| T47D | Cell cycle assay | 24 hrs | Induction of cell cycle arrest in human T47D cells assessed as increase in G0/G1 phase accumulation incubated for 24 hrs by flow cytometry | 28651979 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 8 mg/mL
(18.41 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 434.54 | Formula | C23H30N8O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1211441-98-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LEE011 | Smiles | CN(C)C(=O)C1=CC2=CN=C(N=C2N1C3CCCC3)NC4=NC=C(C=C4)N5CCNCC5 | ||
Mechanism of Action
| Features |
Orally bioavailable CDK4/6-selective inhibitor that has been tested in Phase III clinical trials for treatment of advanced breast cancer.
|
|---|---|
| Targets/IC50/Ki |
CDK4
(Cell-free assay) 10 nM
CDK6
(Cell-free assay) 39 nM
|
| In vitro |
LEE011, as dual CDK4/CDK6 inhibitor, significantly inhibits the growth of 12 of 17 neuroblastoma cell lines with mean IC50 of 307 nM. The growth inhibition of neuroblastoma cell lines is primarily cytostatic and is mediated by a G1 cell-cycle arrest and cellular senescence. |
| In vivo |
LEE011 (200 mg/kg daily, p.o.) significantly causes tumor growth delay in mice harboring the BE2C or 1643 xenografts with no weight loss or other signs of toxicity. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
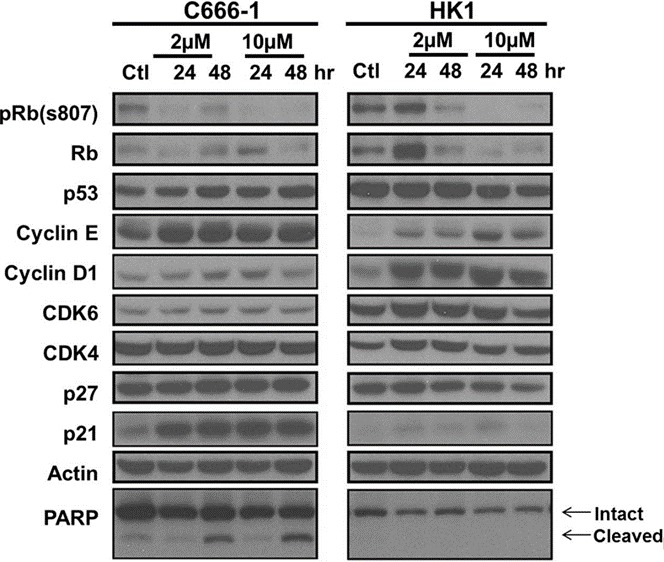
| Western blot | pRb(S807) / Rb / p53 / Cyclin E / Cyclin D1 / CDK4 / CDK6 / p27 / p21 / PARP |
|
29789630 |
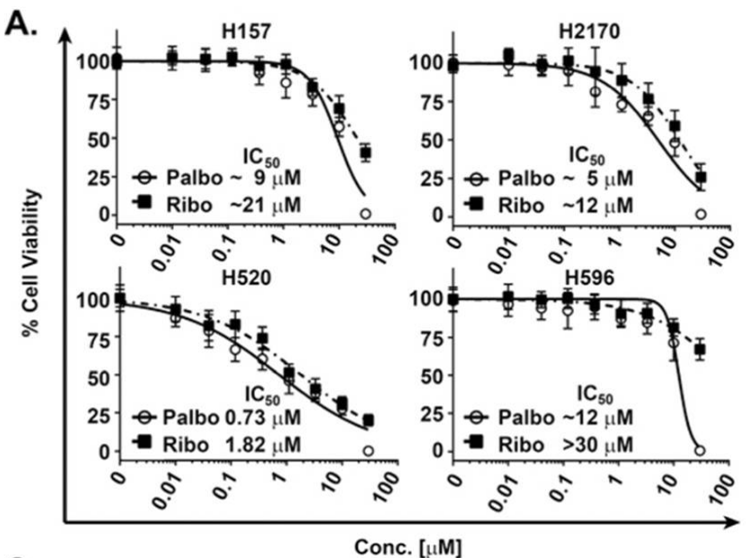
| Growth inhibition assay | Cell viability |
|
26390342 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05843253 | Not yet recruiting | High Grade Glioma|Diffuse Intrinsic Pontine Glioma|Anaplastic Astrocytoma|Glioblastoma|Glioblastoma Multiforme|Diffuse Midline Glioma H3 K27M-Mutant|Metastatic Brain Tumor|WHO Grade III Glioma|WHO Grade IV Glioma |
Nationwide Children''s Hospital|Novartis |
May 30 2024 | Phase 2 |
| NCT06075758 | Recruiting | Breast Cancer |
Novartis Pharmaceuticals|Novartis |
March 7 2024 | -- |
| NCT05996107 | Recruiting | Breast Cancer |
University of Michigan Rogel Cancer Center |
February 27 2024 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































