research use only
Argatroban (MCI-9038) Thrombin inhibitor
Cat.No.S2069
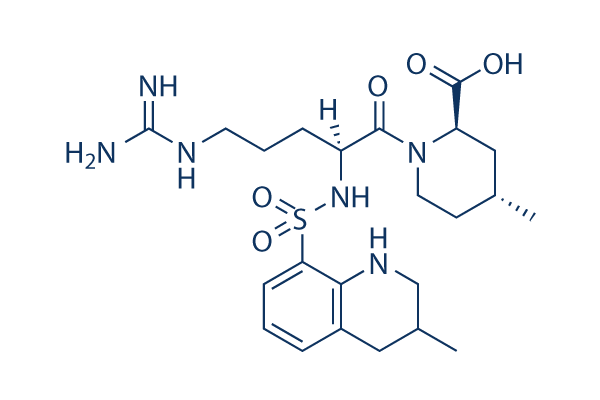
Chemical Structure
Molecular Weight: 508.63
Quality Control
| Related Targets | HDAC Caspase Proteasome Secretase MMP HCV Protease Cysteine Protease Tyrosinase DPP HIV Protease |
|---|---|
| Featured Inhibitors | Y-27632 Dihydrochloride SB431542 CHIR-99021 (Laduviglusib) RMC-7977 RMC-6236 (Daraxonrasib) MRTX1133 MG132 Z-VAD-FMK VT3989 IAG933 |
Solubility
|
In vitro |
DMSO
: 9 mg/mL
(17.69 mM)
Ethanol : 9 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 508.63 | Formula | C23H36N6O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 74863-84-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1CCN(C(C1)C(=O)O)C(=O)C(CCCN=C(N)N)NS(=O)(=O)C2=CC=CC3=C2NCC(C3)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Thrombin
5-39 nM(Ki)
|
|---|---|
| In vitro |
Argatroban (MCI-9038) is a potent and selective synthetic thrombin inhibitor with Ki values against thrombin ranging from 5 nM to 39 nM. It has antithrombotic properties in a wide variety of animal models of both platelet-rich and erythrocyte-rich thrombosis. This compound dose-dependently prevents thrombus formation with an estimated ED50 of 125 μg/kg in this test. It produces dose-dependent increases in the thrombin time with a 511% increase at the highest dose used with only a 73% increase in the APTT at this dose. Argatroban can directly induce phenotype conversion of vascular smooth muscle cells with the resultant up-regulation of SMemb, PAI-1, and beta-actin mRNAs.
|
| In vivo |
Argatroban (MCI-9038) inhibits the formation of microthrombi up to 3 hours after middle cerebral artery (MCA) occlusion; beyond 3 hours, it is ineffective. This compound also significantly reduces the size of ischemic cerebral lesions at 6 hours after MCA occlusion. At a dosage of 0.3 mg/h/rat, it significantly decreases the number of microthrombi 1 day after distal middle cerebral artery (dMCA) occlusion in the rat distal middle cerebral artery occlusion model. Argatroban (0.1 and 0.3 mg/h/rat) significantly reverses a decrease in regional cerebral blood flow (rCBF) 1 day after distal middle cerebral artery (dMCA) occlusion. It (0.3 mg/h/rat) also significantly reduces the size of the cerebral infarction.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06066762 | Not yet recruiting | Heparin-induced Thrombocytopenia |
Assistance Publique - Hôpitaux de Paris |
November 1 2023 | -- |
| NCT05325346 | Completed | Heparin-induced Thrombocytopenia |
Veralox Therapeutics|Celerion |
March 7 2022 | Phase 1 |
| NCT05226442 | Recruiting | Extracorporeal Membrane Oxygenation Complication|Anticoagulants and Bleeding Disorders |
Medical University of Vienna |
December 1 2021 | Phase 2|Phase 3 |
| NCT04751357 | Unknown status | Heparin-induced Thrombocytopenia |
Medical University of Vienna |
August 1 2020 | -- |
| NCT06038682 | Completed | ARDS|COVID-19 |
University Hospital Ostrava |
April 1 2020 | -- |
| NCT02448069 | Completed | Stroke|Cerebral Ischemia |
The University of Texas Health Science Center Houston |
May 2015 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































