research use only
Procarbazine HCl DNA/RNA Synthesis inhibitor
Cat.No.S1995
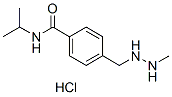
Chemical Structure
Molecular Weight: 257.76
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Solubility
|
In vitro |
Water : 52 mg/mL Ethanol : 52 mg/mL
DMSO
: Insoluble
|
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 257.76 | Formula | C12H19N3O.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 366-70-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC-77213 HCl | Smiles | CC(C)NC(=O)C1=CC=C(C=C1)CNNC.Cl | ||
Mechanism of Action
| In vitro |
Procarbazine plus Cu(II) induce piperidine-labile and formamidopyrimidine-DNA glycosylase-sensitive lesions at the 5'-ACG-3' sequence, complementary to a hotspot of the p53 gene, and the 5'-TG-3' sequence. Procarbazine causes DNA damage through non-enzymatic formation of the Cu(I)-hydroperoxo complex and methyl radicals. Procarbazine has a strong clastogenic effect in hematopoietic cells and is mutagenic in a variety organs after high dose treatment. |
|---|---|
| In vivo |
Procarbazine causes significant decrease in testicular and epididymal weight and a drastic reduction in haploid cells and spermatogenic arrest, demonstrating variation among the test golden hamster. Procarbazine produces a dose-dependent potentiation of MAO A in brown adipose tissue, the elevation being more pronounced following monomethylhydrazine, with activity rising to 350% of that in control homogenates in rats. Procarbazine or monomethylhydrazine reduces metabolism of this amine by a similar degree as had been determined ex-vivo in blood vessel homogenates. Procarbazine is mutagenic, clastogenic and teratogenic in a wide range of test systems of varying complexity and a wide-spectrum carcinogen in rodents and monkeys, causing tumours of the haemopoietic system, the mammary gland, the lung and the nervous system. Procarbazine in vivo undergoes a complex series of metabolic changes that result in the generation of a number of chemically reactive species, including methylating agents and free radicals. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00003564 | Withdrawn | Brain and Central Nervous System Tumors |
M.D. Anderson Cancer Center|National Cancer Institute (NCI) |
Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































