- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Rifapentine DNA/RNA Synthesis inhibitor
Cat.No.S1760
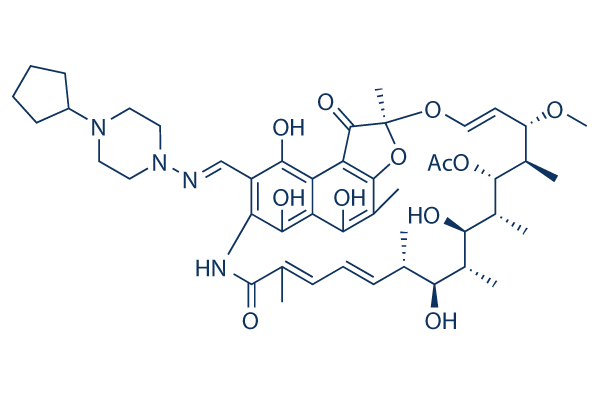
Chemical Structure
Molecular Weight: 877.03
Jump to
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | B02 CX-5461 (Pidnarulex) SCR7 EED226 Favipiravir (T-705) RK-33 Carmofur Triapine (3-AP) BMH-21 YK-4-279 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(114.02 mM)
Ethanol : 10 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 877.03 | Formula | C47H64N4O12 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 61379-65-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MDL473 | Smiles | CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)C | ||
Mechanism of Action
| Targets/IC50/Ki |
DNA-dependent RNA polymerase
|
|---|---|
| In vitro |
Rifapentine inhibits the function of DNA-dependent RNA polymerase in strains of M. tuberculosis, while inducing no effect on mammalian cells. Both this compound and its active metabolite, 25-desacetylrifapentine, localize within monocyte-derived macrophages, thus allowing for intracellular inhibition of M. tuberculosis at a greater kill rate as compared with that of the parent or metabolite alone. It is deacetylated in the liver and induces cytochrome P450 much less than rifampin. This chemical has shown higher bacteriostatic and bactericidal activities (MICs and MBCs) than RMP, especially against intracellular bacteria growing in human monocyte-derived macrophages.
|
| In vivo |
Rifapentine inhibits bacterial RNA synthesis by binding to the β-subunit of DNA-dependent RNA polymerase in susceptible species. This compound is generally more active than rifampicin against sensitive strains of M. tuberculosis. It significantly increases the rate of antipyrine and pentobarbital metabolism in vivo. This chemical also increases liver weight, the content of liver microsomal protein and cytochrome P-450, the activity of NADPH-cytochrome C reductase and NADPH oxidase. When combined with isoniazid (INH) and pyrazinamide (PZA) administered daily, it results in an apparent clearance of M.tuberculosis organisms in the lungs and spleens of infected mice after 10 weeks of treatment.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06281834 | Not yet recruiting | Pediatric HIV Infection|Latent Tuberculosis |
Brigham and Women''s Hospital|APIN Public Health Initiatives|University of Cape Town |
May 2024 | Phase 1 |
| NCT05655702 | Recruiting | Tuberculosis |
ANRS Emerging Infectious Diseases|Haiphong University of Medicine and Pharmacy|Expertise France|Université Montpellier|New York University|CENTER FOR SUPPORTING COMMUNITY DEVELOPMENT INITIATIVES |
October 2 2023 | Not Applicable |
| NCT03730181 | Recruiting | Latent Tuberculosis |
Centers for Disease Control and Prevention|University of Stellenbosch|Johns Hopkins University|Sanofi|University of Cape Town|Chris Hani Baragwanath Academic Hospital|Washington D.C. Veterans Affairs Medical Center |
October 12 2019 | Phase 1|Phase 2 |
| NCT04094012 | Completed | Latent Tuberculosis Infection |
National Taiwan University Hospital |
September 24 2019 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































