research use only
Teriflunomide Dehydrogenase inhibitor
Cat.No.S4169
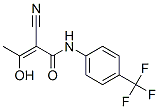
Chemical Structure
Molecular Weight: 270.21
Quality Control
Solubility
|
In vitro |
DMSO
: 27 mg/mL
(99.92 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 270.21 | Formula | C12H9F3N2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 163451-81-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | A77 1726, HMR-1726 | Smiles | CC(=C(C#N)C(=O)NC1=CC=C(C=C1)C(F)(F)F)O | ||
Mechanism of Action
| Targets/IC50/Ki |
dihydroorotate dehydrogenase
|
|---|---|
| In vitro |
Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzyme involved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high-avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus, teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes.
|
| In vivo |
Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological, and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity.
|
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04799288 | Recruiting | HAM/TSP |
National Institute of Neurological Disorders and Stroke (NINDS)|National Institutes of Health Clinical Center (CC) |
September 24 2021 | Phase 1|Phase 2 |
| NCT04129736 | Completed | Multiple Sclerosis Pharmacokinetics |
Jan Lycke|Sahlgrenska University Hospital Sweden |
October 10 2019 | Phase 4 |
| NCT03526224 | Completed | Tecfidera|Teriflunomide |
University at Buffalo |
June 14 2018 | -- |
| NCT03561402 | Completed | Multiple Sclerosis Relapsing-Remitting |
McGill University |
December 1 2016 | -- |
| NCT02833714 | Terminated | RELAPSING REMITTING MULTIPLE SCLEROSIS |
University of North Carolina Chapel Hill|Genzyme a Sanofi Company |
January 2016 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I am planning to use it (S4169) in my in vivo protocol, but I cannot get a homogeneous solution. Could you please provide me with some guidance?
Answer:
This compound is an oral clinical medicine. For oral administration suspension is fine. In paper http://www.pnas.org/content/108/44/18067.full, they also prepared the stock by DMSO and then diluted it into 0.9% sterile saline for animial study.






































