research use only
RRx-001 Dehydrogenase inhibitor
Cat.No.S8405
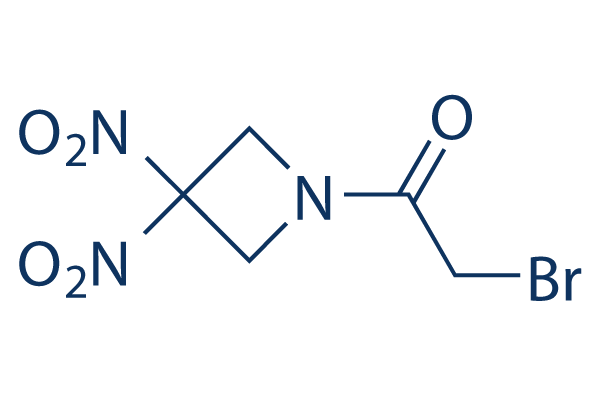
Chemical Structure
Molecular Weight: 268.02
Quality Control
Solubility
|
In vitro |
DMSO
: 54 mg/mL
(201.47 mM)
Ethanol : 14 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 268.02 | Formula | C5H6BrN3O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 925206-65-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1C(CN1C(=O)CBr)([N+](=O)[O-])[N+](=O)[O-] | ||
Mechanism of Action
| Targets/IC50/Ki |
G6PD
Nrf2-ARE
|
|---|---|
| In vitro |
RRx-001 exerts its anti-proliferative effect, at least partially, through interference with glucose 6 phosphate dehydrogenase (G6PD), a key enzyme in the pentose phosphate pathway, responsible for maintaining adequate levels of the major cellular reductant, NADPH. This compound affects glucose and G6PD enzyme activity in three different cancer cell lines namely Hep G2, CACO-2, and HT-29. It induced G6PD inhibition and increased glucose consumption in a concentration dependent fashion. This chemical induces p53 and PARP-1 via generation of ROS/RNS. It exerts anticancer activity, at least in part, by interfering with 3 crucial metabolic demands of rapidly proliferating cells: bioenergetics, macromolecular biosynthesis, and manipulation of cellular cytosolic redox homeostasis. This agent mediates nuclear translocation of Nrf2 and the activation of expression of its downstream enzymes HO-1 and NQO1 in tumor cells. Apart from epigenetic alterations, it acts via pleiotropic mechanisms including redox signaling and redox-induced dysregulation of many different signal pathways such as Nrf2, p53, PARP cleavage, HIF1 alpha, and G6PD activity. It also triggers p53 and p21 activity in response to double-stranded DNA breaks as well as deregulates cancer cellular energetics and metabolism. This compound is a potent activator of the Nrf2-ARE signaling pathway via ROS/RNS generation.
|
| In vivo |
RRx-001 shows promise for short-term blood flow redistribution in tumors with a pericyte- and α-SMA-rich vasculature. This compound monotherapy is well tolerated, with no dose-limiting toxicities. It not only facilitates nuclear translocation of Nrf2, but also upregulates endogenous Nrf2 expression in SCC VII tumors in mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02096341 | Terminated | Malignant Solid Tumor|Lymphomas |
EpicentRx Inc. |
April 2014 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































