research use only
Remibrutinib BTK inhibitor
Cat.No.S9660
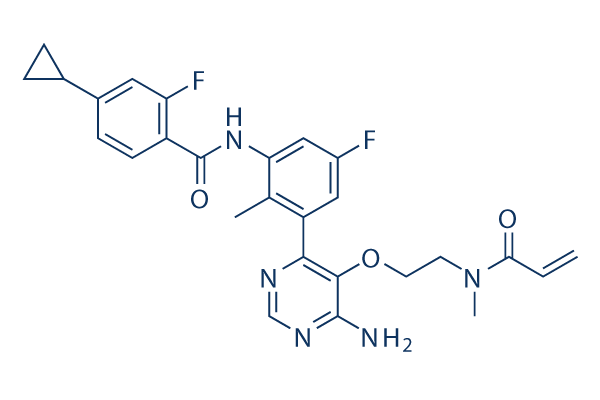
Chemical Structure
Molecular Weight: 507.53
Quality Control
| Related Targets | EGFR VEGFR JAK PDGFR FGFR Src HIF FLT FLT3 HER2 |
|---|---|
| Other BTK Inhibitors | Catadegbrutinib (BGB-16673) Spebrutinib (AVL-292) tirabrutinib(ONO-4059) hydrochloride CGI1746 LFM-A13 Evobrutinib CNX-774 Branebrutinib (BMS-986195) BMS-935177 Fenebrutinib (GDC-0853) |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(197.03 mM)
Ethanol : 4 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 507.53 | Formula | C27H27F2N5O3 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1787294-07-8 | -- | Storage of Stock Solutions |
|
|
| Synonyms | LOU064 | Smiles | CC1=C(C=C(C=C1NC(=O)C2=C(C=C(C=C2)C3CC3)F)F)C4=C(C(=NC=N4)N)OCCN(C)C(=O)C=C | ||
Mechanism of Action
| Targets/IC50/Ki |
BTK
(Cell-free assay) 1.3 nM
IL-8
(Cell-free assay) 2.5 nM
CD69
(Cell-free assay) 18 nM
|
|---|---|
| In vitro |
LOU064 exhibits an exquisite kinase selectivity due to binding to an inactive conformation of BTK and has the potential for a best-in-class covalent BTK inhibitor for the treatment of autoimmune diseases. |
| In vivo |
LOU064 demonstrates potent in vivo target occupancy with an EC90 of 1.6 mg/kg and dose-dependent efficacy in rat collagen-induced arthritis. |
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05677451 | Recruiting | Chronic Spontaneous Urticaria |
Novartis Pharmaceuticals|Novartis |
July 11 2023 | Phase 3 |
| NCT05432388 | Recruiting | Allergy Peanut |
Novartis Pharmaceuticals|Novartis |
October 12 2022 | Phase 2 |
| NCT05156281 | Recruiting | Relapsing Multiple Sclerosis |
Novartis Pharmaceuticals|Novartis |
December 13 2021 | Phase 3 |
| NCT05147220 | Recruiting | Relapsing Multiple Sclerosis |
Novartis Pharmaceuticals|Novartis |
December 16 2021 | Phase 3 |
| NCT04035668 | Terminated | Sjögren Syndrome |
Novartis Pharmaceuticals|Novartis |
July 12 2019 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































