research use only
Ramipril RAAS inhibitor
Cat.No.S1793
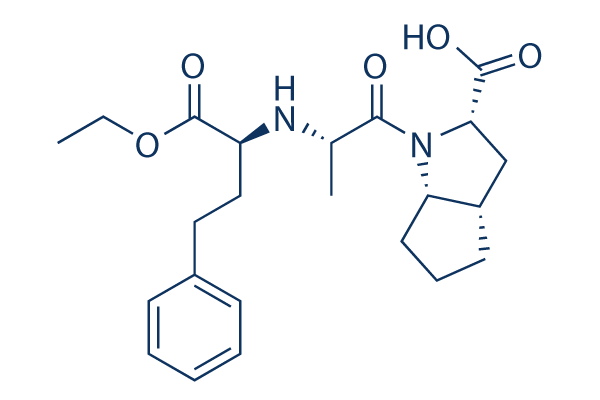
Chemical Structure
Molecular Weight: 416.51
Quality Control
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(199.27 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 416.51 | Formula | C23H32N2O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 87333-19-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HOE-498 | Smiles | CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C3CCCC3CC2C(=O)O | ||
Mechanism of Action
| Features |
A pro-drug converted to its active metabolite, ramiprilat, by liver esterase enzymes.
|
|---|---|
| Targets/IC50/Ki |
ACE
5 nM
|
| In vitro |
Ramipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM. This compound enhances the activity of ACE-associated CK2 and the phosphorylation of ACE Ser1270 in cultured endothelial cells, but is unable to activate JNK or stimulate the nuclear accumulation of c-Jun in endothelial cells expressing a S1270A ACE mutant or in ACE-deficient cells. Prolonged treatment with this chemical increases ACE expression in primary cultures of human endothelial cells and in vivo (mouse lung), which can be prevented by pretreatment with the JNK inhibitor SP600125. It displays little enhanced effect on the rate of in vitro endothelial apoptosis induced by the serum deprivation method. |
| In vivo |
Chronic in vivo administration of Ramipril to rats at a dosage that has similar hypotensive effects in vitro HUVECs significantly reduces the rate of LPS-induced apoptosis compared to the other ACE inhibitors, which contrasts with the apoptosis effect in vitro. This compound inhibits systolic blood pressure (SBP) with IC50 of 1.97 mg/kg in spontaneously hypertensive rats (SHR). When in combination with AT1-receptor blockade by candesartan-cilexetil increases SBP reduction synergistically rather than additively. Administration of this chemical to spontaneously hypertensive rats (SHR) produces significant inhibition of aorta ACE and lung ACE with IC50 ~5 mg/kg, but shows little effect for brain ACE ex vivo. This agent prevents beta cell dysfunction in osteoprotegerin treated mice through decreasing islet monocyte/macrophage infiltration, fibrosis and apoptosis involving decreasing RAS, growth factor genes and inflammatory molecules expressions. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05438316 | Completed | Bioequivalence |
Pharmtechnology LLC|Ligand Research LLC |
June 17 2022 | Phase 1 |
| NCT03475186 | Active not recruiting | Glioblastoma|Radiotherapy; Complications|Cognitive Decline|Chemoradiation |
Wake Forest University Health Sciences|National Cancer Institute (NCI) |
March 25 2019 | Phase 2 |
| NCT03440177 | Recruiting | Obesity Morbid |
Norwegian University of Science and Technology|St. Olavs Hospital|Volvat Medisinsk Senter Stokkan|Namsos Hospital|Alesund Hospital |
January 2 2018 | -- |
| NCT01743014 | Unknown status | Diabetes Type 2|Diabetic Nephropathy|Vascular Disease |
AHEPA University Hospital|Aristotle University Of Thessaloniki |
July 2012 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































