research use only
Leflunomide Dehydrogenase inhibitor
Cat.No.S1247
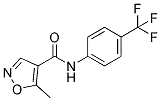
Chemical Structure
Molecular Weight: 270.21
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| B-cells | Function assay | In vitro inhibition of Ab formation from trinitrophenyl-lipopolysaccharide (TNP-LPS) B-cells, IC50=5.1μM | 11384243 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 54 mg/mL
(199.84 mM)
Ethanol : 54 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 270.21 | Formula | C12H9F3N2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 75706-12-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HWA486, RS-34821, SU101 | Smiles | CC1=C(C=NO1)C(=O)NC2=CC=C(C=C2)C(F)(F)F | ||
Mechanism of Action
| Targets/IC50/Ki |
Dihydroorotate dehydrogenase
|
|---|---|
| In vitro |
Leflunomide is actually a prodrug that is processed in vivo to the active metabolite A771726,which has been shown to inhibit proliferation of mononuclear and T-cells. This compound is an inhibitor of several protein tyrosine kinases, with IC50 values between 30 mM and 100 mM in vitro cellular and enzymatic assays. It is capable of inhibiting anti-CD3- and interleukin-2 (IL-2)-stimulated T cell proliferation. This drug is able to inhibit p59fyn and p56lck activity in in vitro tyrosine kinase assays. It also inhibits Ca2+ mobilization in Jurkat cells stimulated by anti-CD3 antibody but not in those stimulated by ionomycin. This compound also inhibits distal events of anti-CD3 monoclonal antibody stimulation, namely, IL-2 production and IL-2 receptor expression on human T lymphocytes. It also inhibits tyrosine phosphorylation in CTLL-4 cells stimulated by IL-2. This immunomodulatory drug may exert its effects by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), which plays a key role in the de novo synthesis of the pyrimidine ribonucleotide uridine monophosphate (rUMP). It prevents the expansion of activated and autoimmune lymphocytes by interfering with the cell cycle progression due to inadequate production of rUMP and utilizing mechanisms involving p53.
|
| In vivo |
Leflunomide is able to prevent and reverse allograft and xenograft rejection in rodents, dogs, and monkeys.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
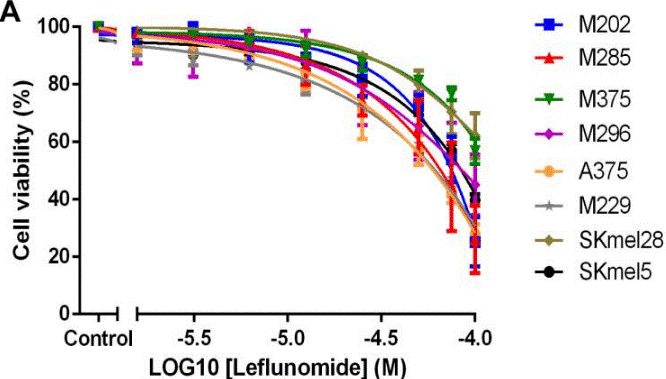
|
29423085 |
| Western blot | DH0DH ATF4 / CHOP CDK2 / Cyclin A2 |
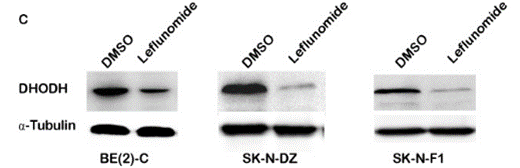
|
23977077 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05605587 | Recruiting | MEN1 Gene Mutation |
University Hospital Basel Switzerland |
May 2 2023 | Not Applicable |
| NCT04212416 | Active not recruiting | Chronic Graft Versus Host Disease|Steroid Refractory Graft Versus Host Disease |
City of Hope Medical Center|National Cancer Institute (NCI) |
May 12 2020 | Phase 1 |
| NCT04361214 | Terminated | COVID-19 |
University of Chicago |
May 5 2020 | Phase 1 |
| NCT03715699 | Unknown status | Autoimmune Disease |
Peking Union Medical College Hospital |
July 1 2018 | Not Applicable |
| NCT03599986 | Completed | Active Rheumatoid Arthritis |
Eva Pharma|DataClin |
June 16 2017 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































