research use only
SRT2104 (GSK2245840) Sirtuin activator
Cat.No.S7792
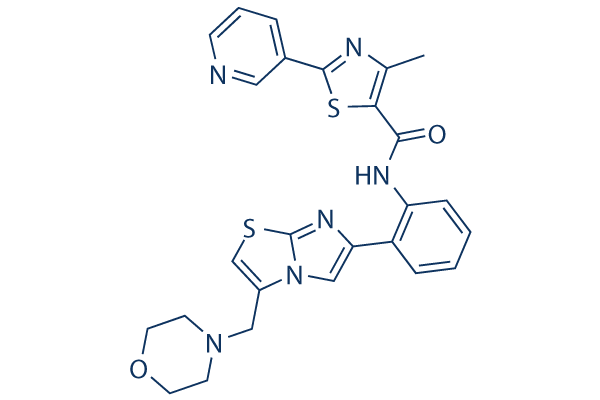
Chemical Structure
Molecular Weight: 516.64
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Casein Kinase eIF |
|---|---|
| Other Sirtuin Inhibitors | SRT1720 HCl Selisistat (EX-527) Sirtinol Fisetin 3-TYP AGK2 OSS_128167 SirReal2 SRT2183 Thiomyristoyl |
Solubility
|
In vitro |
DMSO
: 4 mg/mL
(7.74 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 516.64 | Formula | C26H24N6O2S2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1093403-33-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=C(SC(=N1)C2=CN=CC=C2)C(=O)NC3=CC=CC=C3C4=CN5C(=CSC5=N4)CN6CCOCC6 | ||
Mechanism of Action
| Targets/IC50/Ki |
SIRT1
|
|---|---|
| In vitro |
SRT2104 (GSK2245840) reduces p65/RelA acetylation levels in C2C12 cells.
|
| Kinase Assay |
SIRT1 fluorescence polarization assay and HTS
|
|
In the SIRT1 FP assay for SRT2104 (GSK2245840), SIRT1 activity is monitored using a 20 amino acid peptide (Ac-Glu-Glu-Lys(biotin)-Gly-Gln-Ser-Thr-Ser-Ser-His-Ser-Lys(Ac)-Nle-Ser-Thr-Glu-Gly–Lys(MR121 or Tamra)-Glu-Glu-NH2 ) derived from the sequence of p53. The peptide is N-terminally linked to biotin and C-terminally modified with a fluorescent tag. The reaction for monitoring enzyme activity is a coupled enzyme assay where the first reaction is the deacetylation reaction catalyzed by SIRT1 and the second reaction is cleavage by trypsin at the newly exposed lysine residue. The reaction is stopped and streptavidin is added in order to accentuate the mass differences between substrate and product. The fluorescence polarization reaction conditions are as follows: 0.5 μM peptide substrate, 150 μM βNAD +, 0-10 nM SIRT1, 25 mM Tris-acetate pH 8, 137 mM Na-Ac, 2.7 mM K-Ac, 1 mM Mg-Ac, 0.05% Tween-20, 0.1% Pluronic F127, 10 mM CaCl 2 , 5 mM DTT, 0.025% BSA, and 0.15 mM nicotinamide. The reaction is incubated at 37°C and stopped by addition of nicotinamide, and trypsin is added to cleave the deacetylated substrate. This reaction is incubated at 37 ℃ in the presence of 1 μM streptavidin. Fluorescent polarization is determined at excitation (650 nm) and emission (680 nm) wavelengths.
|
|
| In vivo |
In male C57BL/6J mice, SRT2104 (GSK2245840) (100 mg/kg, p.o.) extends both mean and maximal lifespan of mice fed a standard diet, enhances motor coordination, bone mineral density, and insulin sensitivity, and decreases inflammation. Short-term treatment with this compound preserves bone and muscle mass in an experimental model of atrophy. In Male N171-82Q HD mice, it (diet containing 0.5% SRT2104) effectively penetrates the blood-brain barrier, attenuates brain atrophy, improves motor function, and extends survival.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01453491 | Completed | Colitis Ulcerative |
Sirtris a GSK Company|GlaxoSmithKline |
February 13 2012 | Phase 1 |
| NCT01039909 | Withdrawn | Healthy Volunteer|Atrophy Muscular |
GlaxoSmithKline |
January 2011 | Phase 1 |
| NCT01154101 | Completed | Psoriasis |
Sirtris a GSK Company|GlaxoSmithKline |
June 7 2010 | Phase 2 |
| NCT01031108 | Completed | Diabetes Mellitus Type 2 |
Sirtris a GSK Company|GlaxoSmithKline |
May 28 2010 | Phase 1 |
| NCT01018017 | Completed | Diabetes Mellitus Type 2 |
GlaxoSmithKline |
March 3 2010 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































