
- Inhibitors
- By product type
- Natural Products
- Inducing Agents
- Peptides
- Antibiotics
- Antibody-drug Conjugates(ADC)
- PROTAC
- Hydrotropic Agents
- Dyes
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
By research - Antibodies
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- Featured Products
- MRTX1133
- Nab-Paclitaxel
- KP-457
- IAG933
- RMC-6236 (Daraxonrasib)
- RMC-7977
- Zoldonrasib (RMC-9805)
- GsMTx4
- Navitoclax (ABT-263)
- TSA (Trichostatin A)
- Y-27632 Dihydrochloride
- SB431542
- SB202190
- MK-2206 Dihydrochloride
- LY294002
- Alisertib (MLN8237)
- XAV-939
- CHIR-99021 (Laduviglusib)
- Bafilomycin A1 (Baf-A1)
- Thiazovivin (TZV)
- CP-673451
- Verteporfin
- DAPT
- Galunisertib (LY2157299)
- MG132
- SBE-β-CD
- Tween 80
- Bavdegalutamide (ARV-110)
- Z-VAD-FMK
- Wnt-C59 (C59)
- IWR-1-endo
- (+)-JQ1
- 3-Deazaneplanocin A (DZNep) Hydrochloride
- RepSox (E-616452)
- Erastin
- Q-VD-Oph
- Puromycin Dihydrochloride
- Cycloheximide
- Telaglenastat (CB-839)
- A-83-01
- Ceralasertib (AZD6738)
- Liproxstatin-1
- Emricasan (IDN-6556)
- PMA (Phorbol 12-myristate 13-acetate)
- Dibutyryl cAMP (Bucladesine) sodium
- Nedisertib (M3814)
- PLX5622
- IKE (Imidazole Ketone Erastin)
- STM2457
- Saruparib (AZD5305)
- New Products
- Contact Us
research use only
KW-2449 FLT3 inhibitor
Cat.No.S2158
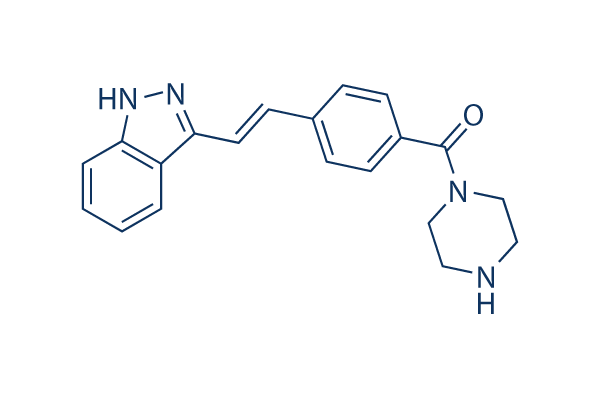
Chemical Structure
Molecular Weight: 332.4
Quality Control
Batch:
Purity:
99.77%
99.77
Chemical Information, Storage & Stability
| Molecular Weight | 332.4 | Formula | C20H20N4O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1000669-72-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN(CCN1)C(=O)C2=CC=C(C=C2)C=CC3=NNC4=CC=CC=C43 | ||
Solubility
|
In vitro |
DMSO
: 67 mg/mL
(201.56 mM)
Ethanol : 67 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
Investigated as a FLT3 inhibitor in clinical trials, with others in early development.
|
|---|---|
| Targets/IC50/Ki |
FLT3 (D835Y) [1]
(Cell-free assay) 1 nM
Abl (T315I) [1]
(Cell-free assay) 4 nM
FLT3 [1]
(Cell-free assay) 6.6 nM
Abl [1]
(Cell-free assay) 14 nM
FGFR1 [1]
(Cell-free assay) 36 nM
Aurora A [1]
(Cell-free assay) 48 nM
JAK2 [1]
(Cell-free assay) 150 nM
Kit [1]
(Cell-free assay) 300 nM
Src [1]
(Cell-free assay) 400 nM
|
| In vitro |
KW-2449, a multikinase inhibitor of FLT3, ABL, ABL-T315I, and Aurora kinase, is under investigation to treat leukemia patients. This compound shows the potent growth inhibitory effects on leukemia cells with FLT3 mutations by inhibition of the FLT3 kinase, resulting in the down-regulation of phosphorylated-FLT3/STAT5, G1 arrest, and apoptosis. Oral administration of this chemical shows dose-dependent and significant tumor growth inhibition in FLT3-mutated xenograft model with minimum bone marrow suppression. In FLT3 wild-type human leukemia, it induces the reduction of phosphorylated histone H3, G2/M arrest, and apoptosis. In Imatinib-resistant leukemia, this agent contributes to release of the resistance by the simultaneous down-regulation of BCR/ABL and Aurora kinases. The inhibitory activity of this compound is not affected by the presence of human plasma protein, such as α1-acid glycoprotein. It has potent growth inhibitory activity against various types of leukemia by several mechanisms of action. This inhibitor has significant activity and warrants clinical study in leukemia patients with FLT3 mutations as well as Imatinib-resistant mutations. Phosphorylation levels of FLT3 and STAT5 are decreased by this chemical in a dose-dependent manner. In addition, it potently inhibits ABL-T315I, which is associated with IM resistance, with IC50 of 4 nM. On the other hand, this compound has little effect on PDGFRβ, IGF-1R, EGFR, and various serine/threonine kinases even at a concentration of 1 μM. It has the potent growth inhibitory activities against not only FLT3/ITD-expressing leukemia cells but also FLT3/KDM-activated and wild-type FLT3-overexpressing leukemia cells. In accordance with growth inhibitory effect, this agent suppresses the phosphorylations of FLT3 (P-FLT3) and its downstream molecule phospho-STAT5 (P-STAT5) in MOLM-13 cells in a dose-dependent manner. Furthermore, it increases the percentage of cells in the G1 phase of the cell cycle and reciprocally reduces the percentage of cells in the S phase, resulting in the increase of apoptotic cell population. This compound can dephosphorylate constitutively active WT-FLT3 kinase but not inhibit the proliferation of leukemia cells if they are not mainly addicted to FLT3 the kinase. [1] It is rapidly absorbed and converted to a major metabolite M1.Preclinical studies reveal that this chemical is converted by monoamine oxidase-B (MAO-B) and aldehyde oxidase into its major metabolite M1.This agent mediates cytotoxicity thru inhibition of FLT3/ITD.It is a direct inhibitor of FLT3 and induces inhibition of its downstream target STAT5. [2] This compound interacts synergistically with HDACIs to induce apoptosis in Ph+ CML cells in a time- and concentration-dependent manner. It synergistically enhances the lethality of vorinostat/SNDX275 in CML cells.This inhibitor regimens are active against additional IM-resistant Bcr/Abl+ leukemia cells. It moderately reduces phosphorylation of histone H3, an indicator of Aurora B activity, in nocodozole-treated K562 cells. [3]
|
| Kinase Assay |
FLT3 phosphorylation
|
|
Leukemia cells are washed in phosphate-buffered saline (PBS), then lysed by resuspending the cells in lysis buffer (20 mM Tris pH 7.4, 100 mM NaCl, 1% Igepal, 1 mM EDTA, 2 mM NaVO4, plus Complete protease inhibitor KW-2449 for 30 minutes while rocking. The extract is clarified by centrifugation at 1.6 × 104 g and the supernatant is assayed for protein (Bio-Rad). A 50-μg aliquot is removed as a whole-cell lysate for analysis of STAT5, and the remainder is used for immunoprecipitation with anti-FLT3. Anti-FLT3 antibody is added to the extract for overnight incubation, then protein A sepharose is added for 2 additional hours. Separate sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE) gels for whole-cell lysate and immunoprecipates are run in parallel. After transfer to Immobilon membranes, immunoblotting is performed with antiphosphotyrosine antibody (4G10) to detect phosphorylated FLT3 or, for the whole-cell lysate gels, with a rat monoclonal antibody against phosphorylated STAT5 (residue Y694) then stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins are visualized using chemiluminescence, exposed on Kodak BioMax XAR film, developed, and scanned using a Bio-Rad GS800 densitometer. The concentration of this compound for which the phosphorylation of FLT3 or STAT5 is inhibited to 50% of its baseline (IC50) is determined using linear regression analysis of the dose response curves. For direct analysis of FLT3 and STAT5 in circulating blasts, peripheral blood is collected in heparinized tubes and promptly chilled on ice. Samples are centrifuged for 10 minutes at 900 g, at 4 °C. The plasma is removed and stored frozen at −80 °C. The buffy coat is carefully transferred to ice-cold PBS, layered onto chilled Ficoll-Hypaque, and centrifuged for 5 minutes at 600 g, at 4 °C. All subsequent steps are carried out at 4 °C. Mononuclear cells are collected and washed rapidly once in red blood cell lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA), then washed once in PBS. Cells are then lysed as described for FLT3 and STAT5 analysis.
|
|
| In vivo |
In the MOLM-13 tumor xenograft model, oral administration of KW-2449 for 14 days shows a potent and significant antitumor effect in a dose-dependent manner. [1]
|
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00346632 | Terminated | Acute Myelogenous Leukemia|Acute Lymphoblastic Leukemia|Myelodysplastic Syndromes|Chronic Myelogenous Leukemia |
Kyowa Kirin Inc. |
June 2006 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































