IκBs are a family of related proteins that have an N-terminal regulatory domain, followed by six or more ankyrin repeats and a PEST domain near their C terminus. IκBα is the best-studied and major IκB protein. [show the full text]
IκB/IKK
| Cat.No. | Product Name | Information | Product Use Citations | Product Validations |
|---|---|---|---|---|
| S8922 | TBK1/IKKε-IN-5 | TBK1/IKKε-IN-5 (compound 1) is a dual inhibitor of TANK-binding kinase 1 (TBK1) and IκB kinase-ε (IKKε/IKK-i) with IC50 of 1.0 nM and 5.6 nM for TBK1 and IKKε, respectively. TBK1/IKKε inhibition enhances response to PD-1 blockade, which effectively predicts tumor response in vivo. |
|
|
| S9042 | Wedelolactone | Wedelolactone, a medicinal plant-derived natural compound, is an inhibitor of IKK that is critical for activation of NF-κB by mediating phosphorylation and degradation of IκBα. This compound is also an inhibitor of caspase-11. |
|
|
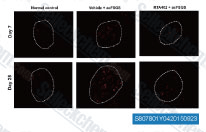
| S8078 | Bardoxolone Methyl (RTA 402) | Bardoxolone Methyl (RTA 402, TP-155, NSC 713200, CDDO Methyl Ester, CDDO-Me) is an IKK inhibitor, showing potent proapoptotic and anti-inflammatory activities; Also a potent Nrf2 activator and nuclear factor-κB (NF-κB) inhibitor. Bardoxolone Methyl abrogates ferroptosis. Bardoxolone methyl induces apoptosis and autophagy in cancer cells. |
|
|
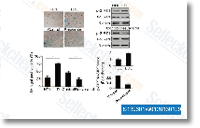
| S1396 | Resveratrol (trans-Resveratrol) | Resveratrol has a wide spectrum of targets including cyclooxygenases(i.e. COX, IC50=1.1 μM), lipooxygenases(LOX, IC50=2.7 μM), kinases, sirtuins and other proteins. It has anti-cancer, anti-inflammatory, blood-sugar-lowering and other beneficial cardiovascular effects. Resveratrol induces mitophagy/autophagy and autophagy-dependent apoptosis. |
|
|
| S2882 | IKK-16 | IKK-16 is a selective IκB kinase (IKK) inhibitor for IKK-2, IKK complex and IKK-1 with IC50 of 40 nM, 70 nM and 200 nM in cell-free assays, respectively. IKK-16 also inhibits LRRK2 Ser935 phosphorylation in cells and LRRK2 kinase activity in vitro with IC50 of 50 nM. |
|

|
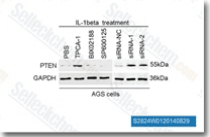
| S2824 | TPCA-1 | TPCA-1 (GW683965) is an inhibitor of IKK-2 with IC50 of 17.9 nM in a cell-free assay, inhibits NF-κB pathway, exhibits 22-fold selectivity over IKK-1. TPCA-1 is also an inhibitor of STAT3 and enhances apoptosis. |
|
|
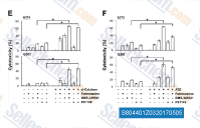
| S8044 | BMS-345541 | BMS-345541 is a highly selective inhibitor of the catalytic subunits of IKK-2 and IKK-1 with IC50 of 0.3 μM and 4 μM in cell-free assays, respectively. |
|
|
| S7352 | Bay 11-7085 | BAY 11-7085 (Bay 11-7083) is an irreversible inhibitor of TNFα-induced IκBα phosphorylation with IC50 of 10 μM. |
|

|
| S2864 | IMD 0354 | IMD-0354 (IKK2 Inhibitor V) is an IKKβ inhibitor and blocks IκBα phosphorylation in NF-κB pathway. |
|
|
| S7948 | MRT67307 HCl | MRT67307 is a potent and dual IKKϵ and TBK1 inhibitor with IC50 of 160 and 19 nM, respectively. MRT67307 potently inhibits ULK1 and ULK2 and blocks autophagy. |
|
|
Signaling Pathway Map
IκB (Inhibitor of κB) functions as a primary inhibitor of NF-κB activation, with an N-terminal regulatory domain, followed by six or more ankyrin repeats and a PEST domain near their C terminus. [1] IκB family contains eight known members, IκBα, IκBβ, IκBε, Bcl-3 (B-cell lymphoma 3), IκBζ, and IκBns (NF-κBδ), as well as the precursor Rel proteins p100 (NF-κB2) and p105 (NF-κB1) due to the presence of multiple ankyrin repeats in their C-terminal halves. IκBα and IκBβ are broadly expressed in all type of cells, whereas IκBε is expressed only in hematopoietic cells. Bcl-3, IκBζ and IkBNS are atypical IκB proteins that exhibit limited expression following NF-κB activation. The regulation of IκB proteins varies by protein type, and each IκB moiety exhibits a unique affinity for NF-κB complexes. [2]
In unstimulated cells, the IκBα proteins mask the nuclear localization signals (NLS) of NF-κB proteins, keeping them sequestered in an inactive state in the cytoplasm. In response to stimuli, IκB kinase (IKK) phosphorylates IκBα leading to the degradation of IκBα, and subsequent NF-κB activation. IκBα expression can be activated by NF-κB to generate a negative feedback loop. Similar to IκBα, IκBβ acts by sequestering p65- and c-Rel-containing complexes in the cytoplasm. However, nuclear localized IκBβ also binds to p65:c-Rel heterodimers, promoting continued binding to specific κB sites, and augmenting late transcription of select target genes (i.e. TNF and IL-1β). IκBε is induced slowly, and selectively regulates p65 homodimers and c-Rel:p65 heterodimers. Bcl-3 functions as a transcriptional co-activator that may both inhibit and facilitate NF-κB-dependent transcription in a context-specific manner. Like Bcl-3, IκBζ can enhance transcription in association with p50 NF-κB dimmers despite the prescence of distinct mechanisms. IκBns selectively inhibits NF-κB-dependent pro-inflammatory gene expression by stabilizing p50 homodimers at κB sites. In addition to exclusively stabilizing RelB dimers, p100 itself can act more broadly in inhibiting NF-κB dimers. The p105 also acts like a typical IκB protein, and is additionally associated with the activation of the MAPK-ERK signaling pathway through the binding of MAP3K8 (TPL2). Moreover, the functions of individual IκB family members are quite heterogeneous and are not limited to this particular role in regulating NF-κB signaling. [2]
In oncology, the direct activation of NF-κB complexes through the loss of the inhibitory proteins IκBα and IκBε has been observed in Hodgkin’s lymphoma. Since the NF-κB signal pathway plays a critical role in tumorigenesis bv way of abberant IκB activity, a variety of compounds targeting IKK and its associated enzymes are in clinical development. [3] For instance, the proteasome inhibitor Bortezomib (Velcade®) has been approved by the FDA for use in haematological malignancies. [4] In addition, Bortezomib is currently being explored in clinical development for its efficacy against solid tumors (clinicaltrials.gov; NCT00479128).





































