research use only
Curcumin p300/CBP inhibitor
Cat.No.S1848
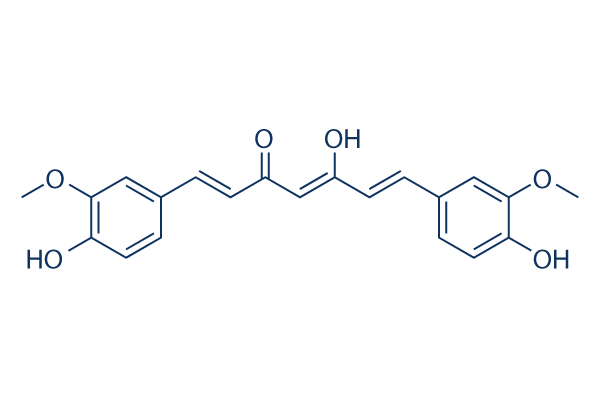
Chemical Structure
Molecular Weight: 368.38
Quality Control
| Related Targets | HDAC JAK BET Histone Methyltransferase PKC PARP HIF PRMT EZH2 AMPK |
|---|---|
| Other p300/CBP Inhibitors | Inobrodib (CCS-1477) A-485 SGC-CBP30 Anacardic Acid CPI-637 E7386 GNE-049 Histone Acetyltransferase Inhibitor II TTK21 I-CBP112 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| U87 | Cell viability assay | 0, 10, 20, 30, 40, and 50 µM/L | 24 hours | At concentrations higher than 20 µM/L, cell viabilities steeply declined | 31062527 | |
| HEL cells | Cell viability assay | 0-30 µmol/L | 24/48 hours | The viability of the HEL cells exposed to curcumin was significantly lower compared to control cells even at low concentrations. | 31033209 | |
| Raw246.7 | Cell viability assay | 1, 5, 10, 50, 100, 200, and 500 g/mL | 31007701 | |||
| RT112 | Cell viability assay | 0.1 or 0.4 μg/ml | 24, 48, and 72 h | Curcumin alone, added at a concentration from 0.1 to 0.4 μg/ml, did not alter cell growth of RT112 cells, slightly reduced UMUC3 cell growth at 0.4 μg/ml, and only moderately suppressed growth of TCCSUP at 0.2 and 0.4 μg/ml, each compared to respective controls | 30984278 | |
| UMUC3 | Cell viability assay | 0.1 or 0.4 μg/ml | 24, 48, and 73 h | Curcumin alone, added at a concentration from 0.1 to 0.4 μg/ml, did not alter cell growth of RT112 cells, slightly reduced UMUC3 cell growth at 0.4 μg/ml, and only moderately suppressed growth of TCCSUP at 0.2 and 0.5 μg/ml, each compared to respective controls | 30984278 | |
| TCCSUP | Cell viability assay | 0.1 or 0.4 μg/ml | 24, 48, and 74 h | Curcumin alone, added at a concentration from 0.1 to 0.4 μg/ml, did not alter cell growth of RT112 cells, slightly reduced UMUC3 cell growth at 0.4 μg/ml, and only moderately suppressed growth of TCCSUP at 0.2 and 0.6 μg/ml, each compared to respective controls | 30984278 | |
| KBM5 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KBM5 cells after 72 hrs by MTT assay, IC50 = 3.84 μM. | 24280069 | ||
| Jurkat | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Jurkat cells after 72 hrs by MTT assay, IC50 = 4.29 μM. | 24280069 | ||
| U266 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U266 cells after 72 hrs by MTT assay, IC50 = 7.57 μM. | 24280069 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 17.3 μM. | 24280069 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells after 24 hrs by MTT assay, IC50 = 32.2 μM. | 26174555 | ||
| SKOV3 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SKOV3 cells after 24 hrs by MTT assay, IC50 = 43.63 μM. | 26174555 | ||
| CT26 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse CT26 cells assessed as inhibition of cell proliferation incubated for 72 hrs by MTS assay, IC50 = 7.7 μM. | 26318057 | ||
| SW620 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW620 cells assessed as inhibition of cell proliferation incubated for 72 hrs by MTS assay, IC50 = 12.52 μM. | 26318057 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells assessed as inhibition of cell proliferation incubated for 72 hrs by MTS assay, IC50 = 24.41 μM. | 26318057 | ||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells assessed as inhibition of cell proliferation incubated for 72 hrs by MTS assay, IC50 = 27.89 μM. | 26318057 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells assessed as inhibition of cell proliferation incubated for 72 hrs by MTS assay, IC50 = 31.39 μM. | 26318057 | ||
| Caco2 | Antiproliferative assay | Antiproliferative activity against human Caco2 cells, IC50 = 10.3 μM. | 26539626 | |||
| U2OS | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U2OS cells expressing wild-type p53 after 48 hrs by crystal violet-staining based spectrophotometric assay, EC50 = 22.8 μM. | 26606246 | ||
| Saos2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Saos2 cells expressing p53 mutant after 48 hrs by crystal violet-staining based spectrophotometric assay, EC50 = 28.1 μM. | 26606246 | ||
| U2OS | Cytotoxicity assay | 24 hrs | Cytotoxicity against human U2OS cells expressing wild-type p53 after 24 hrs by crystal violet-staining based spectrophotometric assay, EC50 = 34.4 μM. | 26606246 | ||
| Saos2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Saos2 cells expressing p53 mutant after 24 hrs by crystal violet-staining based spectrophotometric assay, EC50 = 48.6 μM. | 26606246 | ||
| T98G | Antiproliferative assay | 24 hrs | Antiproliferative activity against human T98G cells after 24 hrs by EZ-Tox assay, IC50 = 10 μM. | 26631318 | ||
| T98G | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T98G cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 25 μM. | 26631318 | ||
| T67 | Neuroprotective assay | Neuroprotective effect in human T67 cells assessed as increase in NQO1 activity using DCIP as substrate | 26696252 | |||
| SH-SY5Y | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SH-SY5Y cells assessed as cell viability after 48 hrs, IC50 = 16.1 μM. | 26705144 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells assessed as cell viability after 48 hrs, IC50 = 21.8 μM. | 26705144 | ||
| HeLa | Cell cycle assay | 16 hrs | Cell cycle arrest in human HeLa cells assessed as accumulation at G1 phase at IC50 to 2 times IC50 after 16 hrs by FACS analysis | 26705144 | ||
| HuH7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HuH7 cells after 48 hrs by MTT assay, IC50 = 48 μM. | 26783179 | ||
| HeLa | Antiproliferative assay | 3 days | Antiproliferative activity against human HeLa cells assessed as inhibition of cell viability after 3 days by WST-1 assay, IC50 = 12.11 μM. | 26827161 | ||
| LNCAP | Antiproliferative assay | 3 days | Antiproliferative activity against human LNCAP cells assessed as inhibition of cell viability after 3 days by WST-1 assay, IC50 = 13.61 μM. | 26827161 | ||
| PC3 | Antiproliferative assay | 3 days | Antiproliferative activity against human PC3 cells assessed as inhibition of cell viability after 3 days by WST-1 assay, IC50 = 25.43 μM. | 26827161 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against human DU145 cells assessed as inhibition of cell viability after 3 days by WST-1 assay, IC50 = 26.23 μM. | 26827161 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production after 24 hrs by Griess assay, IC50 = 19.1 μM. | 26841168 | ||
| BV2 | Function assay | 24 hrs | Inhibition of LPS-induced NO production in mouse BV2 cells assessed as reduction in nitrite level pretreated for 24 hrs before LPS treatment for additional 24 hrs by Griess reaction method, IC50 = 0.52 μM. | 26859776 | ||
| HepG2 | Cytotoxicity assay | Cytotoxicity activity against human HepG2 cells by MTT assay, IC50 = 4.43 μM. | 26873414 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 23.54 μM. | 26873414 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50 = 46.25 μM. | 26873414 | |||
| SKOV3 | Cytotoxicity assay | Cytotoxicity against human SKOV3 cells by MTT assay, IC50 = 49.85 μM. | 26873414 | |||
| A549/CDDP | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549/CDDP cells after 72 hrs by MTT assay, IC50 = 32.39 μM. | 26891099 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 38.12 μM. | 26891099 | ||
| A549/CDDP | Function assay | Inhibition of thioredoxin reductase in human A549/CDDP cells by DTNB dye based microplate spectrophotometry, IC50 = 39.42 μM. | 26891099 | |||
| A549 | Function assay | Inhibition of thioredoxin reductase in human A549 cells by DTNB dye based microplate spectrophotometry, IC50 = 40.56 μM. | 26891099 | |||
| A549 | Apoptosis assay | 2.1 to 4.2 uM | 24 hrs | Induction of apoptosis in human A549 cells at 2.1 to 4.2 uM after 24 hrs by annexin V-FITC/propidium iodide staining-based flow cytometry | 26891099 | |
| A549/CDDP | Apoptosis assay | 0.6 to 1.2 uM | 24 hrs | Induction of apoptosis in human A549/CDDP cells at 0.6 to 1.2 uM after 24 hrs by annexin V-FITC/propidium iodide staining-based flow cytometry | 26891099 | |
| bone marrow | Function assay | 48 hrs | Inhibition of RANKL-induced osteoclast differentiation in ICR mouse bone marrow cells after 48 hrs by TRAP assay, IC50 = 7.5 μM. | 26923696 | ||
| U937 | Function assay | 17 to 20 hrs | Inhibition of LPS/IFN-gamma-induced PGE2 production in PMA-treated human U937 cells after 17 to 20 hrs by enzyme immunoassay, IC50 = 1.88 μM. | 27040659 | ||
| RAW264.7 | Function assay | 17 to 20 hrs | Inhibition of LPS/IFN-gamma-induced PGE2 production in mouse RAW264.7 cells after 17 to 20 hrs by enzyme immunoassay, IC50 = 15.95 μM. | 27040659 | ||
| BV2 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse BV2 cells assessed as inhibition of LPS-induced nitric oxide production after 24 hrs by Griess assay, IC50 = 4.77 μM. | 27228227 | ||
| SH-SY5Y | Neuroprotective assay | 24 hrs | Neuroprotective activity in human SH-SY5Y cells assessed as reduction in 6-OHDA-induced cell death measured after 24 hrs by MTT assay, EC50 = 6.2 μM. | 27420919 | ||
| PC3 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human PC3 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 39 μM. | 27496212 | ||
| DU145 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human DU145 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 41 μM. | 27496212 | ||
| 4T1 | Cytotoxicity assay | 24 hrs | Cytotoxicity against mouse 4T1 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 49.4 μM. | 27496212 | ||
| T47D | Cytotoxicity assay | 24 hrs | Cytotoxicity against human T47D cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 30 μM. | 27517806 | ||
| CHOK1 | Growth inhibition assay | 72 hrs | Growth inhibition of CHOK1 cells assessed as mitochondrial activity measured after 72 hrs by MTT assay, IC50 = 7.5 μM. | 27521589 | ||
| LNCAP | Antiproliferative assay | 3 days | Antiproliferative activity against human LNCAP cells after 3 days by WST or trypan blue assay, IC50 = 13.61 μM. | 27543391 | ||
| PC3 | Antiproliferative assay | 3 days | Antiproliferative activity against human PC3 cells after 3 days by WST or trypan blue assay, IC50 = 25.43 μM. | 27543391 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against human DU145 cells after 3 days by WST or trypan blue assay, IC50 = 26.23 μM. | 27543391 | ||
| BV2 | Function assay | 1 hr | Inhibition of LPS-induced nitric oxide production in mouse BV2 cells pretreated for 1 hr before LPS stimulation measured after 24 hrs by Griess assay, IC50 = 1.42 μM. | 27592135 | ||
| BV2 | Function assay | 1 hr | Inhibition of Src in mouse BV2 cells assessed as suppression of LPS-induced NO release preincubated for 1 hr followed by LPS addition measured after 24 hrs by Griess assay, IC50 = 2.2 μM. | 27617803 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 15.06 μM. | 27816267 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 24.54 μM. | 27816267 | ||
| DU145 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human DU145 cells after 48 hrs by MTT assay, IC50 = 44.71 μM. | 27816267 | ||
| EAhy926 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human EAhy926 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 24.6 μM. | 27843113 | ||
| Caco2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Caco2 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 38.3 μM. | 27843113 | ||
| CHOK1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against CHOK1 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 42 μM. | 27843113 | ||
| SH-SY5Y | Function assay | 1 to 5 uM | 24 hrs | Antioxidant activity against t-BHP-induced oxidative stress in human SH-SY5Y cells assessed as inhibition of ROS production at 1 to 5 uM after 24 hrs by DCFH-DA probe based flow cytometric analysis | 27856236 | |
| NCI-H1650 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1650 cells measured after 72 hrs by MTT assay, IC50 = 20.7 μM. | 27886548 | ||
| LLC | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse LLC cells measured after 72 hrs by MTT assay, IC50 = 24.7 μM. | 27886548 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells measured after 72 hrs by MTT assay, IC50 = 27.8 μM. | 27886548 | ||
| K562 | Function assay | 48 hrs | Inhibition of P-gp in doxorubicin resistant human K562 cells assessed as reduction in cell viability measured after 48 hrs by Presto blue assay, GI50 = 19.67 μM. | 27908756 | ||
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells measured after 48 hrs by Presto blue assay, GI50 = 21.67 μM. | 27908756 | ||
| H460 | Function assay | 48 hrs | Inhibition of P-gp in drug resistant human H460 cells assessed as reduction in cell viability measured after 48 hrs by sulforhodamine B assay, GI50 = 26 μM. | 27908756 | ||
| NCI-H460 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H460 cells measured after 48 hrs by sulforhodamine B assay, GI50 = 27.3 μM. | 27908756 | ||
| K562 | Function assay | 20 to 30 uM | 48 hrs | Down regulation of p-gp expression in doxorubicin resistant human K562 cells at 20 to 30 uM measured after 48 hrs by Western blot method | 27908756 | |
| K562 | Cell cycle assay | 20 uM | 48 hrs | Cell cycle arrest in doxorubicin resistant human K562 cells assessed as decrease in G0/G1 phase at 20 uM measured after 48 hrs by propidium iodide staining based flow cytometry | 27908756 | |
| K562 | Cell cycle assay | 20 uM | 48 hrs | Cell cycle arrest in doxorubicin resistant human K562 cells assessed as increase in G2/M phase at 20 uM measured after 48 hrs by propidium iodide staining based flow cytometry | 27908756 | |
| K562 | Function assay | 20 to 40 uM | 48 hrs | Up regulation of p53 expression in doxorubicin resistant human K562 cells at 20 to 40 uM measured after 48 hrs by Western blot method | 27908756 | |
| K562 | Cell cycle assay | 20 uM | 48 hrs | Cell cycle arrest in doxorubicin resistant human K562 cells assessed as decrease in S phase at 20 uM measured after 48 hrs by propidium iodide staining based flow cytometry | 27908756 | |
| K562 | Cell cycle assay | 30 to 40 uM | 48 hrs | Cell cycle arrest in doxorubicin resistant human K562 cells assessed as accumulation at G0/G1 phase at 30 to 40 uM measured after 48 hrs by propidium iodide staining based flow cytometry | 27908756 | |
| K562 | Cell cycle assay | 30 to 40 uM | 48 hrs | Cell cycle arrest in doxorubicin resistant human K562 cells assessed as decrease in G2/M phase at 30 to 40 uM measured after 48 hrs by propidium iodide staining based flow cytometry | 27908756 | |
| K562 | Function assay | 20 to 40 uM | 48 hrs | Down regulation of cyclin B1 expression in doxorubicin resistant human K562 cells at 20 to 40 uM measured after 48 hrs by Western blot method | 27908756 | |
| K562 | Apoptosis assay | 20 to 40 uM | 48 hrs | Induction of apoptosis in doxorubicin resistant human K562 cells decrease in pro-caspase 3 level at 20 to 40 uM measured after 48 hrs by Western blot method | 27908756 | |
| K562 | Apoptosis assay | 20 to 40 uM | 48 hrs | Induction of apoptosis in doxorubicin resistant human K562 cells assessed as increase in PARP-1 cleavage at 20 to 40 uM measured after 48 hrs by Western blot method | 27908756 | |
| Raji | Function assay | 48 hrs | Inhibition of TPA-induced EBV-early antigen activation in human Raji cells after 48 hrs by indirect immunofluorescence method, IC50 = 12.1 μM. | 27933896 | ||
| BV2 | Function assay | 24 hrs | Inhibition of LPS-induced nitric oxide production in mouse BV2 cells pretreated for 24 hrs followed by LPS-stimulation after 24 hrs by Griess assay, IC50 = 2.36 μM. | 28032759 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 12.22 μM. | 28038323 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 15.81 μM. | 28038323 | ||
| BT549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BT549 cells after 48 hrs by MTT assay, IC50 = 24.93 μM. | 28038323 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50 = 25.41 μM. | 28038323 | ||
| DU145 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human DU145 cells after 48 hrs by MTT assay, IC50 = 28.41 μM. | 28038323 | ||
| BV2 | Function assay | 30 mins | Inhibition of LPS-induced nitric oxide production in mouse BV2 cells pretreated for 30 mins followed by LPS-stimulation after 24 hrs by Griess assay, IC50 = 4.5 μM. | 28055210 | ||
| THP1 | Function assay | 1 hr | Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs using factor 10a chromogenic substrate in presence of prothrombin complex, IC50 = 0.01316 μM. | 28109788 | ||
| RAW264.7 | Antiinflammatory assay | 20 uM | Anti-inflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced IL-6 production at 20 uM by ELISA | 28284806 | ||
| RAW264.7 | Antiinflammatory assay | 20 uM | Anti-inflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced TNFalpha production at 20 uM by ELISA | 28284806 | ||
| PC12 | Neuroprotective assay | 0.1 to 10 uM | 24 hrs | Neuroprotective activity against H202-induced toxicity in rat PC12 cells assessed as increase in cell viability at 0.1 to 10 uM after 24 hrs by MTT assay | 28302511 | |
| PC12 | Neuroprotective assay | 0.1 to 10 uM | 24 hrs | Neuroprotective activity against Abeta42-induced toxicity in rat PC12 cells assessed as increase in cell viability at 0.1 to 10 uM after 24 hrs by MTT assay | 28302511 | |
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells up to 72 hrs by MTT assay, IC50 = 13.77 μM. | 28319780 | |||
| MDA-MB-231 | Antiproliferative assay | Antiproliferative activity against human MDA-MB-231 cells up to 72 hrs by MTT assay, IC50 = 16.23 μM. | 28319780 | |||
| PC3 | Antiproliferative assay | Antiproliferative activity against human PC3 cells up to 72 hrs by MTT assay, IC50 = 18.8 μM. | 28319780 | |||
| BT474 | Antiproliferative assay | 24 hrs | Antiproliferative activity against HER2 positive human BT474 cells after 24 hrs by MTT assay, IC50 = 30.14 μM. | 28319780 | ||
| MDA-MB-453 | Antiproliferative assay | 24 hrs | Antiproliferative activity against HER2 positive human MDA-MB-453 cells after 24 hrs by MTT assay, IC50 = 32.4 μM. | 28319780 | ||
| MDA-MB-157 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human MDA-MB-157 cells after 24 hrs by MTT assay, IC50 = 40.38 μM. | 28319780 | ||
| SKBR3 | Antiproliferative assay | 24 hrs | Antiproliferative activity against HER2 positive human SKBR3 cells after 24 hrs by MTT assay, IC50 = 42.83 μM. | 28319780 | ||
| MCF7 | Antiproliferative assay | 24 hrs | Antiproliferative activity against ER/PR positive human MCF7 cells after 24 hrs by MTT assay, IC50 = 42.89 μM. | 28319780 | ||
| MDA-MB-468 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human MDA-MB-468 cells after 24 hrs by MTT assay, IC50 = 42.89 μM. | 28319780 | ||
| T47D | Antiproliferative assay | 24 hrs | Antiproliferative activity against ER/PR positive human T47D cells after 24 hrs by MTT assay, IC50 = 47.91 μM. | 28319780 | ||
| cells at | Growth inhibition assay | 30 uM | 48 hrs | Growth inhibition of human Hep3B cells at 30 uM after 48 hrs by MTT assay | 28342400 | |
| PWR-1E | Antiproliferative assay | 3 days | Antiproliferative activity against human PWR-1E cells after 3 days by WST-1 assay, IC50 = 8.14 μM. | 28388523 | ||
| LNCAP | Antiproliferative assay | 3 days | Antiproliferative activity against human LNCAP cells after 3 days by WST-1 assay, IC50 = 13.61 μM. | 28388523 | ||
| PC3 | Antiproliferative assay | 3 days | Antiproliferative activity against human PC3 cells after 3 days by WST-1 assay, IC50 = 25.43 μM. | 28388523 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against human DU145 cells after 3 days by WST-1 assay, IC50 = 26.23 μM. | 28388523 | ||
| HepG2 | Function assay | 5 uM | 24 hrs | Activation of Nrf2 in human HepG2 cells assessed as cytoprotection against t-BHP-induced oxidative cell death at 5 uM incubated for 24 hrs followed by compound removal with subsequent incubation with 900 uM of t-BHP for 6 hrs by MTT assay | 28399452 | |
| BV2 | Antineuroinflammatory assay | 15 mins | Antineuroinflammatory activity in mouse BV2 cells assessed as inhibition of LPS-induced nitric oxide production pretreated for 15 mins followed by LPS-stimulation measured after 24 hrs by colorimetric method, IC50 = 6 μM. | 28514148 | ||
| L428 | Function assay | 2 hrs | Inhibition of NF-kappaB (unknown origin) expressed in human L428 cells after 2 hrs by luciferase reporter gene assay | 28526369 | ||
| PWR-1E | Antiproliferative assay | 3 days | Antiproliferative activity against human PWR-1E cells expressing androgen receptor and PSA assessed as decrease in cell viability after 3 days by WST assay, IC50 = 8.85 μM. | 28601720 | ||
| LNCAP | Antiproliferative assay | 3 days | Antiproliferative activity against human LNCAP cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 13.61 μM. | 28601720 | ||
| PC3 | Antiproliferative assay | 3 days | Antiproliferative activity against human PC3 cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 25.43 μM. | 28601720 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against human DU145 cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 26.23 μM. | 28601720 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 12 μM. | 28654265 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 14 μM. | 28654265 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 15 μM. | 28654265 | ||
| LN229 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human LN229 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 15 μM. | 28654265 | ||
| U87 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U87 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 16 μM. | 28654265 | ||
| COLO201 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO201 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 18 μM. | 28654265 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 18 μM. | 28654265 | ||
| T47D | Cytotoxicity assay | 48 hrs | Cytotoxicity against human T47D cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 19 μM. | 28654265 | ||
| MML1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MML1 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 19 μM. | 28654265 | ||
| BxPC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BxPC3 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 29 μM. | 28654265 | ||
| SK-MEL-2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SK-MEL-2 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 36 μM. | 28654265 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.5 μM. | 28716495 | ||
| HCT116 | Function assay | 48 hrs | Induction of mitochondrial membrane potential loss in human HCT116 cells at antiproliferative GI50 after 48 hrs by JC-1 staining based fluorescence assay | 28716495 | ||
| HCT116 | Function assay | 60 mins | Induction of mitochondrial superoxide generation in human HCT116 cells at antiproliferative GI50 after 60 mins by mitoSOX red-probe based fluorescence assay | 28716495 | ||
| HCT116 | Function assay | 60 mins | Induction of reactive oxygen species generation in human HCT116 cells at antiproliferative GI50 after 60 mins by DCFH-DA probe based assay | 28716495 | ||
| HCT116 | Function assay | 500 uM | 48 hrs | Induction of glutathione oxidation in human HCT116 cells assessed as increase in GSSG/GSH ratio at 500 uM in presence of NADPH after 48 hrs by Ellman's method | 28716495 | |
| MCF7 | Function assay | 48 hrs | Modulation activity at ER in human MCF7 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 48 μM. | 28797797 | ||
| A549 | Growth inhibition assay | Growth inhibition of human A549 cells, IC50 = 23 μM. | 28814374 | |||
| BGC823 | Growth inhibition assay | 20 uM | 72 hrs | Growth inhibition of human BGC823 cells at 20 uM after 72 hrs by MTT assay | 28881286 | |
| SGC7901 | Growth inhibition assay | 20 uM | 72 hrs | Growth inhibition of human SGC7901 cells at 20 uM after 72 hrs by MTT assay | 28881286 | |
| MFC | Growth inhibition assay | 20 uM | 72 hrs | Growth inhibition of MFC cells at 20 uM after 72 hrs by MTT assay | 28881286 | |
| BV2 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity against mouse BV2 cells assessed as inhibition of LPS-induced nitric oxide production after 24 hrs by griess assay, IC50 = 4 μM. | 29035525 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 17.14 μM. | 29174816 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 18.71 μM. | 29174816 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 33.12 μM. | 29174816 | ||
| HepG2 | Function assay | 5 hrs | Activation of Nrf2 (unknown origin) expressed in human HepG2 cells after 5 hrs by ARE-driven luciferase reporter gene assay, EC50 = 21 μM. | 29223100 | ||
| BGC823 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BGC823 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 19.5 μM. | 29288946 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 20.3 μM. | 29288946 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 26.2 μM. | 29288946 | ||
| NCI-H460 | Growth inhibition assay | 5 to 10 uM | 24 to 72 hrs | Growth inhibition of human NCI-H460 cells assessed as reduction in cell viability at 5 to 10 uM incubated for 24 to 72 hrs by MTT assay | 29288946 | |
| NCI-H460 | Growth inhibition assay | 20 uM | 18 hrs | Anti-growth activity against human NCI-H460 cells assessed as reduction in colony formation at 20 uM after 18 hrs by crystal violet-based colony forming assay | 29288946 | |
| NCI-H460 | Antimigratory assay | 20 uM | 48 hrs | Anti-migratory activity against human NCI-H460 cells at 20 uM incubated for 48 hrs by wound healing assay | 29288946 | |
| NCI-H460 | Apoptosis assay | 15 mg/kg | 15 days | Induction of apoptosis in tumor of human NCI-H460 cells xenografted in BALB/C nu nude mouse assessed as downregulation of bcl2 expression at 15 mg/kg, ip administered everyday for 15 days by Western blot analysis | 29288946 | |
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 13.78 μM. | 29421568 | ||
| THP1 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human THP1 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 22.54 μM. | 29421568 | ||
| K562 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human K562 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 23.12 μM. | 29421568 | ||
| LO2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human LO2 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 25.67 μM. | 29421568 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 31.89 μM. | 29421568 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 17.11 μM. | 29429834 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 18.65 μM. | 29429834 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 33.15 μM. | 29429834 | ||
| MCF7 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human MCF7 cells preincubated for 4 hrs followed by incubation in compound free media for 24 hrs by MTT assay, IC50 = 22.1 μM. | 29605808 | ||
| WRL68 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human WRL68 cells preincubated for 4 hrs followed by incubation in compound free media for 24 hrs by MTT assay, IC50 = 27.8 μM. | 29605808 | ||
| A549 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human A549 cells preincubated for 4 hrs followed by incubation in compound free media for 24 hrs by MTT assay, IC50 = 43.9 μM. | 29605808 | ||
| NCI-H1975 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1975 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 1.4 μM. | 29655083 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 6.8 μM. | 29655083 | ||
| HL7702 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL7702 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 7.2 μM. | 29655083 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 8.5 μM. | 29655083 | ||
| NCI-H1650 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1650 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 20.5 μM. | 29655083 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 10 μM. | 29798827 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 10.91 μM. | 29886021 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 16.01 μM. | 29909338 | ||
| SMMC7721 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SMMC7721 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 19.47 μM. | 29909338 | ||
| QGY7703 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human QGY7703 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 21.44 μM. | 29909338 | ||
| HHL5 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HHL5 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 22.27 μM. | 29909338 | ||
| LO2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human LO2 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 23.87 μM. | 29909338 | ||
| Vero | Cytotoxicity assay | Cytotoxicity against African green monkey Vero cells assessed as growth inhibition, CC50 = 36.8 μM. | 29920454 | |||
| HT22 | Cytotoxicity assay | 24 hrs | Cytotoxicity against mouse HT22 cells assessed as reduction in cell viability after 24 hrs by MTT assay, MNTD = 1 μM. | 30031653 | ||
| HT22 | Cytoprotection assay | 1 uM | 24 hrs | Cytoprotection against glutamate-induced apoptosis in mouse HT22 cells assessed as decrease in Bax/Bcl2 ratio at 1 uM after 24 hrs by RT-PCR method | 30031653 | |
| HT22 | Cytoprotection assay | 1 uM | 24 hrs | Cytoprotection against glutamate-induced oxidative stress in mouse HT22 cells assessed as decrease in iNOS mRNA level at 1 uM after 24 hrs by RT-PCR method | 30031653 | |
| HT22 | Cytoprotection assay | 1 uM | 24 hrs | Cytoprotection against glutamate-induced excitotoxicity in mouse HT22 cells assessed as increase in cell viability at 1 uM after 24 hrs by optical microscopic assay | 30031653 | |
| HT22 | Cytoprotection assay | 1 uM | 24 hrs | Cytoprotection against glutamate-induced excitotoxicity in mouse HT22 cells assessed as decrease in glutamate-induced cell cycle arrest at sub-G1 phase at 1 uM after 24 hrs by propidium iodide staining based flow cytometry | 30031653 | |
| HT22 | Cytoprotection assay | 1 uM | 24 hrs | Cytoprotection against glutamate-induced excitotoxicity in mouse HT22 cells assessed as increase in cell proliferation at 1 uM after 24 hrs by MTT assay | 30031653 | |
| LNCAP | Antiproliferative assay | Antiproliferative activity against androgen-sensitive human LNCAP cells, IC50 = 2 μM. | 30121214 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against androgen-insensitive human DU145 cells, IC50 = 2 μM. | 30121214 | |||
| PC3 | Antiproliferative assay | Antiproliferative activity against androgen-insensitive human PC3 cells, IC50 = 2 μM. | 30121214 | |||
| LNCAP | Antiproliferative assay | 3 days | Antiproliferative activity against androgen-sensitive human LNCAP cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 13.61 μM. | 30121214 | ||
| PC3 | Antiproliferative assay | 3 days | Antiproliferative activity against androgen-insensitive human PC3 cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 25.43 μM. | 30121214 | ||
| DU145 | Antiproliferative assay | 3 days | Antiproliferative activity against androgen-insensitive human DU145 cells assessed as decrease in cell viability after 3 days by WST assay, IC50 = 26.23 μM. | 30121214 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 9.8 μM. | 30138803 | ||
| EJ28 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EJ28 cells after 72 hrs by MTT assay, IC50 = 10.5 μM. | 30138803 | ||
| HT1080 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT1080 cells after 72 hrs by MTT assay, IC50 = 13.1 μM. | 30138803 | ||
| SW620 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW620 cells after 72 hrs by MTT assay, IC50 = 14 μM. | 30138803 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 17.9 μM. | 30138803 | ||
| SMMC7721 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SMMC7721 cells assessed as reduction in cell viability incubated for 24 hrs by MTT assay, IC50 = 16.68 μM. | 30771605 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability incubated for 24 hrs by MTT assay, IC50 = 17.37 μM. | 30771605 | ||
| LO2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human LO2 cells assessed as reduction in cell viability incubated for 24 hrs by MTT assay, IC50 = 22.69 μM. | 30771605 | ||
| QGY7703 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human QGY7703 cells assessed as reduction in cell viability incubated for 24 hrs by MTT assay, IC50 = 23.91 μM. | 30771605 | ||
| HHL5 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HHL5 cells assessed as reduction in cell viability incubated for 24 hrs by MTT assay, IC50 = 24.19 μM. | 30771605 | ||
| HCT-116 | Function assay | 24 hrs | Inhibition of chymotrypsin-like activity of human 26S proteasome in human HCT-116 cells assessed as decrease in AMC hydrolysis using Suc-LLVY-AMC as substrate preincubated for 24 hrs followed by addition of substrate and measured after 2 hrs by fluorometr, IC50 = 10 μM. | 30776692 | ||
| SW480 | Function assay | 24 hrs | Inhibition of chymotrypsin-like activity of human 26S proteasome in human SW480 cells assessed as decrease in AMC hydrolysis using Suc-LLVY-AMC as substrate preincubated for 24 hrs followed by addition of substrate and measured after 2 hrs by fluorometric, IC50 = 10 μM. | 30776692 | ||
| RAW264.7 | Function assay | 10 uM | 0.5 hrs | Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-induced TNFalpha production at 10 uM pretreated for 0.5 hrs followed by LPS challenge and measured after 24 hrs by ELISA relative to control | 30780088 | |
| RAW264.7 | Function assay | 0.5 hrs | Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-induced TNFalpha production pretreated for 0.5 hrs followed by LPS challenge and measured after 24 hrs by ELISA | 30780088 | ||
| RAW264.7 | Function assay | 0.5 hrs | Inhibition of LPS-induced NFkappaB p65 nuclear translocation in ICR mouse RAW264.7 cells preincubated for 0.5 hrs followed by LPS challenge and measured after 40 mins by DAPI staining-based immunofluorescence microscopic analysis | 30780088 | ||
| RAW264.7 | Function assay | 10 uM | 0.5 hrs | Inhibition of LPS-induced p38 MAPK phosphorylation in ICR mouse RAW264.7 cells at 10 uM preincubated for 0.5 hrs followed by LPS challenge and measured after 30 mins by immunoblotting analysis | 30780088 | |
| RAW264.7 | Function assay | 10 uM | 0.5 hrs | Inhibition of LPS-induced ERK phosphorylation in ICR mouse RAW264.7 cells at 10 uM preincubated for 0.5 hrs followed by LPS challenge and measured after 30 mins by immunoblotting analysis | 30780088 | |
| RAW264.7 | Function assay | 10 uM | 0.5 hrs | Inhibition of NFkappaB/MAPK signaling in LPS-induced mouse RAW264.7 cells assessed as down regulation of IkappaB expression at 10 uM preincubated for 0.5 hrs followed by LPS-challenge and measured after 30 mins by immunoblotting analysis | 30780088 | |
| RAW264.7 | Function assay | 10 uM | 0.5 hrs | Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-induced IL6 production at 10 uM pretreated for 0.5 hrs followed by LPS challenge and measured after 24 hrs by ELISA relative to control | 30780088 | |
| RAW264.7 | Function assay | 0.5 hrs | Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-induced IL6 production pretreated for 0.5 hrs followed by LPS challenge and measured after 24 hrs by ELISA | 30780088 | ||
| RAW264.7 | Function assay | 4 hrs | Inhibition of RANKL-induced osteoclastogenesis in murine RAW264.7 cells assessed as formation of TRAP positive multinucleated osteoclasts pretreated for 4 hrs followed by RANKL addition measured after 72 hrs, IC50 = 3 μM. | 30794412 | ||
| BV2 | Antineuroinflammatory assay | 1 hr | Anti-neuroinflammatory activity in human BV2 cells assessed as inhibition of LPS-induced NO production preincubated for 1 hr followed by LPS stimulation measured after 24 hrs by Griess reagent based assay, IC50 = 1.58 μM. | 31005056 | ||
| HeLa | Anticancer assay | Anticancer activity against human HeLa cells, IC50 = 4.33 μM. | 31129455 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells, IC50 = 6.3 μM. | 31129455 | |||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 Cells after 48 hrs by Cell Titer Blue assay, IC50 = 6.7 μM. | 31129455 | ||
| HSC4 | Cytotoxicity assay | Cytotoxicity against human HSC4 cells, IC50 = 7.1 μM. | 31129455 | |||
| U87MG | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U87MG Cells 48 hrs by MTS assay, IC50 = 7.15 μM. | 31129455 | ||
| HSC2 | Cytotoxicity assay | Cytotoxicity against human HSC2 cells, IC50 = 7.8 μM. | 31129455 | |||
| LX2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human LX2 Cells after 48 hrs by MTT assay, IC50 = 9.14 μM. | 31129455 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 Cells after 48 hrs by MTT assay, IC50 = 9.28 μM. | 31129455 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 Cells after 48 hrs by MTT assay, IC50 = 9.44 μM. | 31129455 | ||
| 3T3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse 3T3 Cells after 48 hrs by MTT assay, IC50 = 9.51 μM. | 31129455 | ||
| AGS | Cytotoxicity assay | Cytotoxicity against human AGS Cells, IC50 = 9.77 μM. | 31129455 | |||
| HSC3 | Cytotoxicity assay | Cytotoxicity against human HSC3 cells, IC50 = 11 μM. | 31129455 | |||
| MDA-MB-231 | Anticancer assay | 72 hrs | Anticancer activity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 11.45 μM. | 31129455 | ||
| SMMC7721 | Anticancer assay | 72 hrs | Anticancer activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 12.57 μM. | 31129455 | ||
| LX2 | Anticancer assay | 72 hrs | Anticancer activity against human LX2 cells after 72 hrs by MTT assay, IC50 = 13.97 μM. | 31129455 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa Cells, IC50 = 14.03 μM. | 31129455 | |||
| SW480 | Cytotoxicity assay | 24 to 48 hrs | Cytotoxicity against human SW480 Cells after 24 to 48 hrs by MTT assay, IC50 = 15 μM. | 31129455 | ||
| U251MG | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U251MG cells after 48 hrs by MTS assay, IC50 = 15.26 μM. | 31129455 | ||
| RWPE1 | Cytotoxicity assay | Cytotoxicity against human RWPE1 cells, IC50 = 15.62 μM. | 31129455 | |||
| MCF7 | Apoptosis assay | Induction of apoptosis in human MCF7 cells, IC50 = 17.1 μM. | 31129455 | |||
| BGC823 | Cytotoxicity assay | Cytotoxicity against human BGC823 cells, IC50 = 17.5 μM. | 31129455 | |||
| BxPC3 | Cytotoxicity assay | Cytotoxicity against human BxPC3 cells, IC50 = 18.25 μM. | 31129455 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 Cells, IC50 = 18.74 μM. | 31129455 | |||
| H1299 | Cytotoxicity assay | Cytotoxicity against human H1299 cells, IC50 = 18.93 μM. | 31129455 | |||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells, IC50 = 19.98 μM. | 31129455 | |||
| EAhy926 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EAhy926 Cells after 72 hrs by MTT assay, IC50 = 20 μM. | 31129455 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 Cells after 72 hrs by MTT assay, IC50 = 20.3 μM. | 31129455 | ||
| AGS | Cytotoxicity assay | Cytotoxicity against human AGS Cells by MTT assay, IC50 = 20.76 μM. | 31129455 | |||
| PC3 | Anticancer assay | Anticancer activity against human PC3 cells, IC50 = 20.9 μM. | 31129455 | |||
| MGC803 | Cytotoxicity assay | Cytotoxicity against human MGC803 cells, IC50 = 21.2 μM. | 31129455 | |||
| CHOK1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CHOK1 Cells after 72 hrs by MTT assay, IC50 = 21.3 μM. | 31129455 | ||
| Hep2 | Cytotoxicity assay | Cytotoxicity against human Hep2 Cells, IC50 = 21.37 μM. | 31129455 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, IC50 = 22.5 μM. | 31129455 | |||
| SGC7901 | Cytotoxicity assay | Cytotoxicity against human SGC7901 cells, IC50 = 22.5 μM. | 31129455 | |||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 Cells after 72 hrs by MTT assay, IC50 = 23.4 μM. | 31129455 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 Cells by MTT assay, IC50 = 25.33 μM. | 31129455 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells, IC50 = 26.5 μM. | 31129455 | |||
| A549 | Cytotoxicity assay | 24 to 48 hrs | Cytotoxicity against human A549 Cells after 24 to 48 hrs by MTT assay, IC50 = 28 μM. | 31129455 | ||
| Caco2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Caco2 Cells after 72 hrs by MTT assay, IC50 = 28 μM. | 31129455 | ||
| GES-1 | Cytotoxicity assay | Cytotoxicity against human GES-1 cells, IC50 = 29.1 μM. | 31129455 | |||
| DU145 | Anticancer assay | Anticancer activity against human DU145 cells, IC50 = 31.7 μM. | 31129455 | |||
| MDCK2 | Cytotoxicity assay | 15 mins | Cytotoxicity against mitoxantrone resistant human MDCK2 cells expressing BCRP after 15 mins by fluorescence assay, IC50 = 32 μM. | 31129455 | ||
| HL-7702 | Cytotoxicity assay | Cytotoxicity against human HL-7702 cells, IC50 = 32.33 μM. | 31129455 | |||
| K562 | Cytotoxicity assay | 24 to 48 hrs | Cytotoxicity against human K562 Cells after 24 to 48 hrs by MTT assay, IC50 = 36 μM. | 31129455 | ||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 Cells, IC50 = 36.77 μM. | 31129455 | |||
| GBM1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human GBM1 Cells 48 hrs by MTS assay, IC50 = 39.65 μM. | 31129455 | ||
| PC12 | Cytotoxicity assay | 24 hrs | Cytotoxicity against rat PC12 cells measured after 24 hrs by WST8/PMS assay, CC50 = 42 μM. | 31262559 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50 = 10.5 μM. | 31326241 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells, IC50 = 8.2 μM. | 31336310 | |||
| B16 | Cytotoxicity assay | Cytotoxicity against human B16 cells by MTT assay, IC50 = 8.5 μM. | 31336310 | |||
| HT1080 | Function assay | Decrease in UPA expression in human HT1080 cells, IC50 = 10 μM. | 31336310 | |||
| Hep3B | Cytotoxicity assay | Cytotoxicity against human Hep3B cells, IC50 = 10 μM. | 31336310 | |||
| BT474 | Cytotoxicity assay | Cytotoxicity against human BT474 cells, IC50 = 11 μM. | 31336310 | |||
| T47D | Cytotoxicity assay | Cytotoxicity against human T47D cells, IC50 = 11 μM. | 31336310 | |||
| HaCaT | Cytotoxicity assay | Cytotoxicity against human HaCaT cells, IC50 = 13 μM. | 31336310 | |||
| KM12 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human KM12 cells after 4 hrs by MTT assay, IC50 = 16 μM. | 31336310 | ||
| HPL1D | Cytotoxicity assay | Cytotoxicity against human HPL1D cells, IC50 = 16 μM. | 31336310 | |||
| SW480 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human SW480 cells after 4 hrs by MTT assay, IC50 = 17.94 μM. | 31336310 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, IC50 = 19.9 μM. | 31336310 | |||
| HT-29 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human HT-29 cells after 4 hrs by MTT assay, IC50 = 20.73 μM. | 31336310 | ||
| WiDr | Cytotoxicity assay | 4 hrs | Cytotoxicity against human WiDr cells after 4 hrs by MTT assay, IC50 = 26.82 μM. | 31336310 | ||
| SW116 | Cytotoxicity assay | 4 hrs | Cytotoxicity against human SW116 cells after 4 hrs by MTT assay, IC50 = 27.46 μM. | 31336310 | ||
| Colon 26 | Cytotoxicity assay | 4 hrs | Cytotoxicity against mouse Colon 26 cells after 4 hrs by MTT assay, IC50 = 30.34 μM. | 31336310 | ||
| 22Rv1 | Function assay | Inhibition of NFkappaB p65 nuclear translocation in human 22Rv1 cells at GI50 by DAPI staining based immunofluorescence assay | 31767266 | |||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity human PC3 cells assessed as reduction in Ki67 protein level at GI50 incubated for 48 hrs by DAPI staining based immunofluorescence assay | 31767266 | ||
| 22Rv1 | Antiproliferative assay | 48 hrs | Antiproliferative activity human 22Rv1 cells assessed as reduction in Ki67 protein level at GI50 incubated for 48 hrs by DAPI staining based immunofluorescence assay | 31767266 | ||
| PC3 | Function assay | Inhibition of NFkappaB p65 nuclear translocation in human PC3 cells at GI50 by DAPI staining based immunofluorescence assay | 31767266 | |||
| PC3 | Function assay | Inhibition of NFkappaB p65 transcriptional activity in human PC3 cells at GI50 by sandwich ELISA | 31767266 | |||
| 22Rv1 | Function assay | Inhibition of NFkappaB p65 transcriptional activity in human 22Rv1 cells at GI50 by sandwich ELISA | 31767266 | |||
| Sf21 | Function assay | 10 mins | Inhibition of recombinant human full-length His6-tagged p300 expressed in baculovirus infected Sf21 insect cells using [3H]acetylCoA as substrate preincubated for 10 mins followed by substrate addition and measured after 10 mins by liquid scintillation co, IC50 = 25 μM. | 31910017 | ||
| COS7 | Function assay | 10 mins | Inhibition of rabbit SERCA1b expressed in COS7 cells microsomal membranes preincubated for 10 mins followed by addition of ATP and measured after 40 mins by ELISA method, Ki = 5.8 μM. | 32030976 | ||
| COS7 | Function assay | 10 mins | Inhibition of human SERCA3a expressed in COS7 cells microsomal membranes preincubated for 10 mins followed by addition of ATP and measured after 40 mins by ELISA method, Ki = 8.6 μM. | 32030976 | ||
| A549 | Function assay | 15 mins | Inhibition of microsomal PGES1 in ILbeta-stimulated human A549 cells using PGH2 as substrate preincubated for 15 mins followed by substrate addition measured after 1 min by RP-HPLC analysis, IC50 = 0.2 μM. | ChEMBL | ||
| RAW264.7 | Function assay | 20 hrs | Inhibition of LPS-induced nitric oxide production in mouse RAW264.7 cells measured after 20 hrs by griess assay, IC50 = 0.8 μM. | ChEMBL | ||
| MDCK | Antiviral assay | 40 hrs | Antiviral activity against Influenza A virus (A/WSN/33(H1N1)) infected in MDCK cells assessed as inhibition of virus-induced cytopathic effect incubated for 40 hrs by CellTiter-Glo assay, IC50 = 6.7 μM. | ChEMBL | ||
| MDCK | Antiviral assay | 40 hrs | Antiviral activity against Influenza A virus (A/WSN/33(H1N1)) infected in MDCK cells assessed as reduction in virus-induced cytopathic effect after 40 hrs by CellTiter-Glo assay, IC50 = 6.7 μM. | ChEMBL | ||
| RAW264.7 | Antiinflammatory assay | 17 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS/IFN-gamma-stimulated nitric oxide production after 17 hrs by Griess assay, IC50 = 14.7 μM. | ChEMBL | ||
| HEK293T | Cytotoxicity assay | Cytotoxicity against HEK293T cells, IC50 = 15.2 μM. | ChEMBL | |||
| J774.1 | Cytotoxicity assay | Cytotoxicity against mouse J774.1 cells, IC50 = 31 μM. | ChEMBL | |||
| MDCK | Cytotoxicity assay | 40 hrs | Cytotoxicity against MDCK cells assessed as decrease in cell viability after 40 hrs by CellTiter-Glo assay, CC50 = 48.3 μM. | ChEMBL | ||
| MDCK | Cytotoxicity assay | 40 hrs | Cytotoxicity against MDCK cells assessed as reduction in cell viability after 40 hrs by CellTiter-Glo assay, CC50 = 48.3 μM. | ChEMBL | ||
| 3T3L1 | Function assay | 10 ng/ml | Inhibition of TNFalpha-induced NFkappaB activation (unknown origin) expressed in mouse 3T3L1 cells at 10 ng/ml by luciferase reporter gene assay | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 250 mg/mL
(678.64 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 368.38 | Formula | C21H20O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 458-37-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Diferuloylmethane, Natural Yellow 3, Turmeric yellow | Smiles | COC1=C(C=CC(=C1)C=CC(=O)CC(=O)C=CC2=CC(=C(C=C2)O)OC)O | ||
Mechanism of Action
| Targets/IC50/Ki |
Nrf2
(Cell-free assay) Ferroptosis
HDAC
(Cell-free assay) NF-κB
(Cell-free assay) p300
(Cell-free assay) ~25 μM
|
|---|---|
| In vitro |
Curcumin induces the expression of forkhead box protein O1 (FOXO1) through activation of extracellular signal-regulated kinase 1/2 signaling. This compound inhibits cell proliferation, which was associated with upregulation of the cyclin-dependent kinase inhibitors, p27 and p21, and downregulation of cyclin D1. It induces endoplasmic reticulum (ER) stress and mitochondrial dysfunction as evidenced by up-regulation of CCAAT/enhancer binding protein homologous protein (CHOP), phosphorylation of JNK and down-regulation of SERCA2ATPase, release of cytochrome c, decrease of Bcl-2 and reduction of mitochondrial membrane potential in both AGS and HT-29 cells. |
| In vivo |
Chronic treatment with curcumin significantly reverses the CMS-induced behavioral abnormalities in stressed rats. Additionally, this compound effectively inhibits cytokine gene expression at both the mRNA and the protein level and reduces the activation of NF-κB. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-IRE1α / IREα HDAC1/ HDAC2 p-STAT3 / STAT3 |
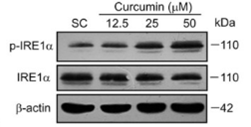
|
29901626 |
| Immunofluorescence | β-catenin PKD1 EGFR / LC3 |
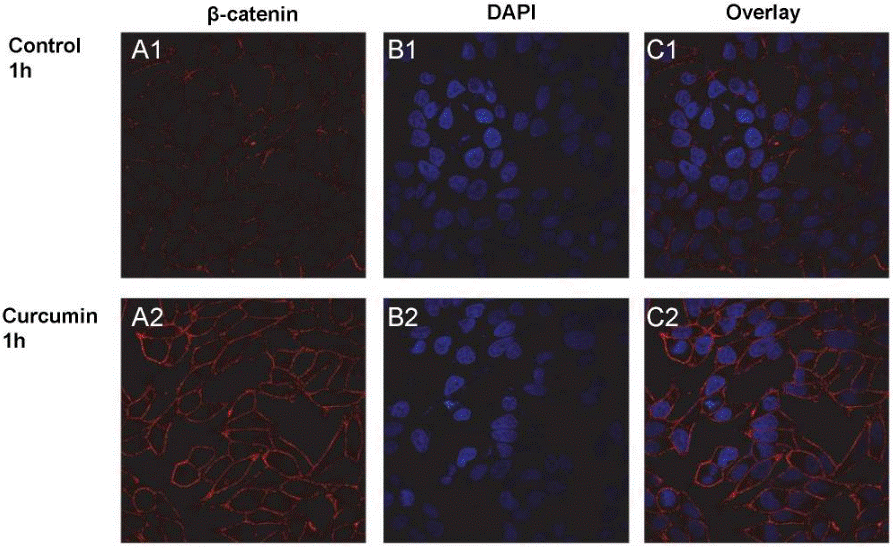
|
22523587 |
| Growth inhibition assay | Cell proliferation Cell viability |
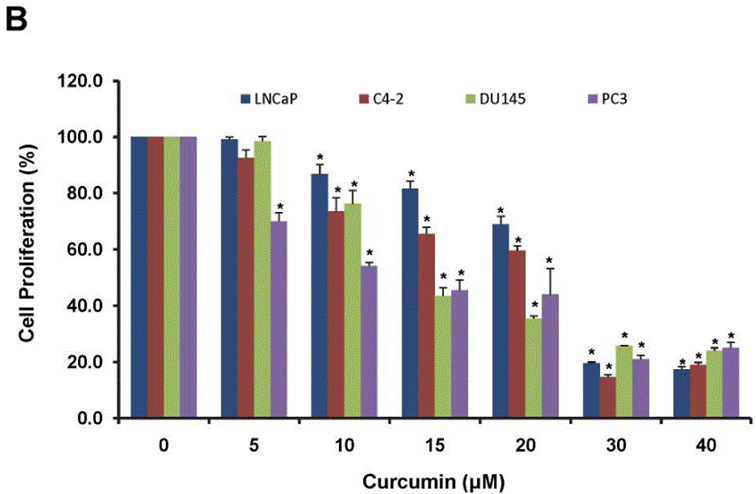
|
22523587 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06053411 | Not yet recruiting | Interaction |
Washington State University |
January 1 2024 | Early Phase 1 |
| NCT05947513 | Not yet recruiting | Cervical Cancer |
Addis Ababa University|Center for Innovative Drug Development and Therapeutic Trials for Africa Addis Ababa University|Akay Natural Ingredients Private Limited |
October 2023 | Phase 1|Phase 2 |
| NCT05966441 | Not yet recruiting | Chemotherapy-induced Peripheral Neuropathy |
Ain Shams University |
August 30 2023 | Phase 2 |
| NCT05774704 | Recruiting | Bioavailability|Gut Microbiome|Safety |
Texas Tech University Health Sciences Center |
August 21 2023 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































