research use only
Rofecoxib COX inhibitor
Cat.No.S3043
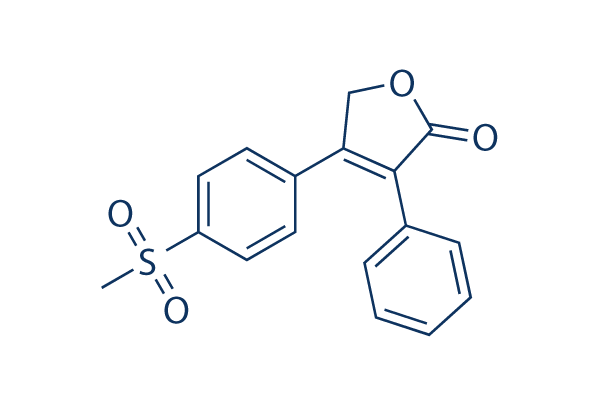
Chemical Structure
Molecular Weight: 314.36
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| J774 | Function assay | In vitro inhibitory activity against Prostaglandin G/H synthase 2 in murine J774 cells, IC50=0.012 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 63 mg/mL
(200.4 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 314.36 | Formula | C17H14O4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 162011-90-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MK-0966 | Smiles | CS(=O)(=O)C1=CC=C(C=C1)C2=C(C(=O)OC2)C3=CC=CC=C3 | ||
Mechanism of Action
| Targets/IC50/Ki |
COX-2
18 nM
|
|---|---|
| In vitro |
Rofecoxib inhibits the COX-2-dependent production of PGE2 in human osteosarcoma cells with an IC50 of 26 nM. This compound is a time-dependent inhibitor of purified human recombinant COX-2 with an IC50 of 0.34 μM. It causes inhibition of purified human COX-1 in a non-time-dependent manner. In a human whole blood assay, this chemical selectively inhibits lipopolysaccharide-induced, COX-2-derived PGE2 synthesis with an IC50 value of 0.53 μM compared with an IC50 value of 18.8 μM for the inhibition of COX-1-derived thromboxane B2 synthesis after blood coagulation. It moderately inhibits phenacetin O-deethylation with an IC50 of 23 μM. And a 30-minute preincubation with microsomes and NADPH considerably increases the inhibitory effect of this compound with an IC50 of 4.2 μM. Inactivation of CYP1A2 by rofecoxib requires NADPH, and is characterized by a K i of 4.8 μM.
|
| Kinase Assay |
In vitro biochemical and pharmacological assaysinhibition studies with recombinant human COX-1 and COX-2
|
|
Microsomal preparations of recombinant human COX-1 and COX-2 are prepared from a vaccinia virus-COS-7 cell expression system. Recombinant human COX-1 and COX-2 are expressed in baculovirus-Sf9 cells, and enzymes are purified. Enzymatic activity is monitored continuously by either a fluorescence assay measuring the appearance of the oxidized form of the reducing agent cosubstrate homovanillic acid or by oxygen consumption. The HPLC assay for the assessment of inhibition of purified COX-1 by this compound with 0.1 μM arachidonic acid substrate concentration, the determination of the stoichiometry of the complex between COX-2 and this chemical, the dissociation rate constant of the enzyme-inhibitor complex by recovery of enzymatic activity, and the recovery of intact this compound from that complex are all performed as described previously. The solvent system for the HPLC analysis of this chemical is 15:85 MeOH/aqueous potassium phosphate (1 g/liter), with elution by a linear gradient of 15 to 75% MeOH over 25 minutes with detection at 275 nm on a Novapak C18 column.
|
|
| In vivo |
Rofecoxib potently inhibits carrageenan-induced paw edema, carrageenan-induced paw hyperalgesia, lipopolysaccharide-induced pyresis with IC50 of 1.5 mg/kg, 1.0 mg/kg and 0.24 mg/kg, respectively. This compound also blocks adjuvant-induced arthritis with an IC50 of 0.74 mg/kg/day. It also has a protective effect on adjuvant-induced destruction of cartilage and bone structures in rats. Oral administration of this chemical decreases portal pressure in rats that are treated with CCl4 for 8 weeks. In addition, its administration reduces the number of activated HSCs and downregulates hepatic protein levels of three detected types of collagen, laminin, VEGF and CTGF in CCl4-treated rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00637988 | Completed | Barrett''s Esophagus |
AstraZeneca |
April 2002 | Phase 4 |
| NCT00038389 | Terminated | Glioma|Brain Neoplasms |
M.D. Anderson Cancer Center |
October 2001 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































