research use only
Omaveloxolone (RTA-408) NRF2 activator
Cat.No.S7672
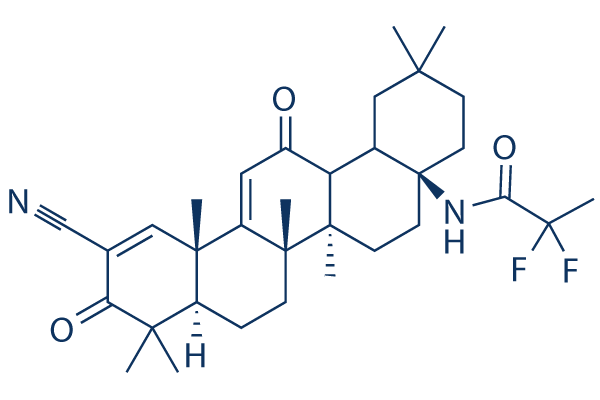
Chemical Structure
Molecular Weight: 554.71
Quality Control
| Related Targets | HDAC Antioxidant ROS IκB/IKK Nrf2 AP-1 MALT NOD |
|---|---|
| Other NF-κB Inhibitors | DCZ0415 BAY 11-7082 (BAY 11-7821) JSH-23 QNZ (EVP4593) Caffeic Acid Phenethyl Ester SC75741 DHA (Dihydroartemisinin) Withaferin A (WFA) Andrographolide Evodiamine |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(180.27 mM)
Ethanol : 3 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 554.71 | Formula | C33H44F2N2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1474034-05-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1(CCC2(CCC3(C(C2C1)C(=O)C=C4C3(CCC5C4(C=C(C(=O)C5(C)C)C#N)C)C)C)NC(=O)C(C)(F)F)C | ||
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
Nrf2
|
|---|---|
| In vitro |
RTA 408 potently increases expression of Nrf2 target genes and reverses IFNγ-mediated suppression of Gclc expression in RAW 264.7 cells. In a panel of eight human tumor cell lines, RTA 408 inhibits growth with an average GI50 value of 260 nM and induces apoptosis. RTA 408 also inhibits NF-κB and activates JNK in tumor cells.
|
| In vivo |
In mice with radiation-induced dermatitis, 1.0% RTA 408 markedly reduces epidermal and collagen thickening, prevents dermal necrosis and completely alleviates skin ulcers. In rat skin, RTA 408 activates Nrf2 and induces cytoprotective genes. RTA 408 also mitigates hematopoietic acute radiation syndrome in mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06054893 | Not yet recruiting | Friedreich Ataxia |
Biogen |
November 1 2023 | Phase 1 |
| NCT05909644 | Completed | Healthy Adult Subjects |
Reata a wholly owned subsidiary of Biogen|Celerion|Q2 Solutions|Altasciences Company Inc.|Biogen |
July 5 2023 | Phase 1 |
| NCT03902002 | Completed | Hepatic Impairment |
Reata a wholly owned subsidiary of Biogen|Biogen |
July 19 2019 | Phase 1 |
| NCT03931590 | Completed | Healthy Male Subjects |
Reata a wholly owned subsidiary of Biogen|Biogen |
April 11 2019 | Phase 1 |
| NCT03664453 | Completed | Healthy |
Reata a wholly owned subsidiary of Biogen|Biogen |
October 29 2018 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































