research use only
Naquotinib(ASP8273) EGFR inhibitor
Cat.No.S8412
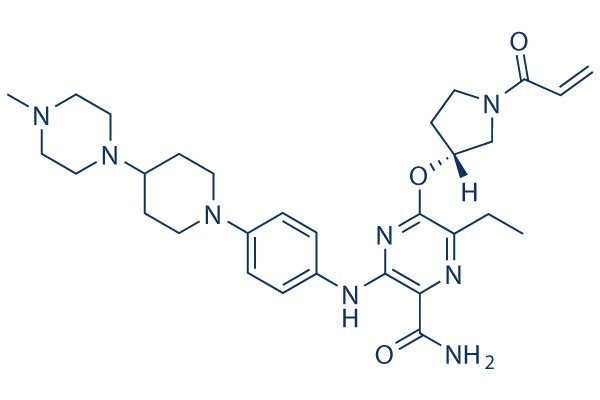
Chemical Structure
Molecular Weight: 562.71
Quality Control
| Related Targets | VEGFR JAK PDGFR FGFR Src HIF FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other EGFR Inhibitors | Lazertinib (YH25448) Icotinib Hydrochloride Sunvozertinib AG-490 AG-1478 Canertinib (CI-1033) WZ4002 Poziotinib (NOV120101, HM781-36B) Rociletinib (CO-1686) Genistein |
Solubility
|
In vitro |
Ethanol : 100 mg/mL
DMSO
: 52 mg/mL
(92.4 mM)
Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 562.71 | Formula | C30H42N8O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1448232-80-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCC1=C(N=C(C(=N1)C(=O)N)NC2=CC=C(C=C2)N3CCC(CC3)N4CCN(CC4)C)OC5CCN(C5)C(=O)C=C | ||
Mechanism of Action
| Targets/IC50/Ki |
mutant EGFR
|
|---|---|
| In vitro |
Naquotinib (ASP8273) is a small-molecule irreversible TKI inhibitor that inhibits the kinase activity of EGFR mutations including T790M, with limited activity against EGFR wild-type (WT) NSCLC. In the in vitro enzymatic and cell-based assays, this compound is evaluated against EGFR mutants (L858R, exon 19 deletion, L858R/T790M, and del19/T790M) and WT EGFR. It is found by mass spectrometry to covalently bind to a mutant EGFR (L858R/T790M) via cysteine residue 797 in the kinase domain of EGFR with long-lasting inhibition of EGFR phosphorylation for 24 h. In the NSCLC cell lines harboring the above EGFR mutations, it has IC50 values of 8-33 nM toward EGFR mutants, more potently than that of WT EGFR (IC50 value of 230 nM). It is further shown to suppress the signaling pathway through ERK and Akt. This compound even shows activity in mutant EGFR cell line which is resistant to other EGFR TKIs including AZD9291 and CO-1686.
|
| In vivo |
In mouse xenograft studies, S8412 induces tumor regression in NCI-H1975 (L858R/T790M), HCC827 (del ex19) and PC-9 (del ex19) xenograft models by repeated oral dosing in a dose-dependent manner. Dosing schedules do not affect the efficacy of ASP8273. In HCC827 and NCI-H1975 xenograft models, S8412 induces tumor regression at 10, 30 and 100mg/kg without affecting body weight. S8412 also produces tumor growth inhibition from 10mg/kg in the NSCLC patient derived tumor xenograft, LU1868 which express T790M/L858R. On the other hand, S8412 does not produce significant tumor growth inhibition at 10 and 30mg/kg in A431 xenograft model.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03082300 | Terminated | Non-small Cell Lung Cancer (NSCLC)|Epidermal Growth Factor Receptor (EGFR) Mutations |
Astellas Pharma Global Development Inc.|Astellas Pharma Inc |
March 24 2017 | Phase 1 |
| NCT02674555 | Withdrawn | Epidermal Growth Factor Receptor (EGFR) Mutations|Solid Tumors |
Astellas Pharma Global Development Inc.|Astellas Pharma Inc |
November 15 2016 | Phase 1 |
| NCT02500927 | Terminated | EGFR-TKI-naïve Patients With NSCLC Harboring EGFR Activating Mutations |
Astellas Pharma Inc |
June 25 2015 | Phase 2 |
| NCT02113813 | Completed | Non-Small-Cell Lung Cancer (NSCLC)|Epidermal Growth Factor Receptor Mutations |
Astellas Pharma Global Development Inc.|Astellas Pharma Inc |
April 9 2014 | Phase 1 |
| NCT02192697 | Terminated | Non-small Cell Lung Cancer |
Astellas Pharma Inc |
January 23 2014 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































