research use only
Canertinib (CI-1033) EGFR inhibitor
Cat.No.S1019
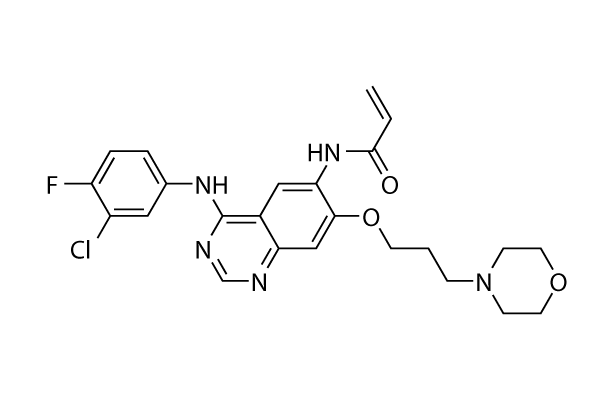
Chemical Structure
Molecular Weight: 485.94
Quality Control
| Related Targets | VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other EGFR Inhibitors | Sunvozertinib Icotinib Hydrochloride AG-490 AG-1478 Rociletinib (CO-1686) WZ4002 Genistein Poziotinib (NOV120101, HM781-36B) PD153035 PD153035 HCl |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human HCC827 cells | Proliferation assay | 72 h | Antiproliferative activity against human HCC827 cells harboring EGFR del E746-A750 mutant after 72 hrs by MTS assay, IC50=0.001 μM | |||
| A431 cells | Function assay | Inhibition of EGF-stimulated autophosphorylation of EGFR enzyme in A431 cells detected by immunoblotting, IC50=0.0074 μM | ||||
| MDA-MB 453 cells | Function assay | Inhibition of autophosphorylation of ERBB2 receptor kinase in MDA-MB 453 cells, IC50=0.009 μM | ||||
| human BT474 cells | Proliferation assay | 3 days | Antiproliferative activity against human BT474 cells overexpressing ERBb2 after 3 days by methylene blue staining, EC50=0.01 μM | |||
| mouse BAF3 cells | Function assay | Inhibition of Blk expressed in mouse BAF3 cells assessed as cytotoxicity, IC50=0.029 μM | ||||
| human HN5 cells | Proliferation assay | 3 days | Antiproliferative activity against human HN5 cells overexpressing EGFR after 3 days by methylene blue staining, EC50=0.05 μM | |||
| human NCI-H1975 cells | Proliferation assay | 72 h | Antiproliferative activity against human NCI-H1975 cells harboring EGFR L858R/T790M mutant after 72 hrs by MTS assay, IC50=0.064 μM | |||
| human A431 cells | Proliferation assay | 72 h | Antiproliferative activity against human A431 cells overexpressing EGFR after 72 hrs by MTS assay, IC50=0.15 μM | |||
| human A549 cells | Proliferation assay | 72 h | Antiproliferative activity against human A549 cells expressing wild type EGFR coexpressing k-Ras mutant after 72 hrs by MTS assay, IC50=1.59 μM | |||
| mouse BAF3 cells | Function assay | Inhibition of JAK3 expressed in mouse BAF3 cells assessed as cytotoxicity, IC50=2 μM | ||||
| human HL7702 cells | Proliferation assay | 72 h | Antiproliferative activity against human HL7702 cells expressing wilt type EGFR after 72 hrs by MTS assay, IC50=2.3 μM | |||
| human A431 cells | Function assay | 1 μM | 1 h | Irreversible inhibition of EGFR autophosphorylation in human A431 cells at 1 uM incubated for 1 hr followed by compound wash out measured 5 hrs post EGF addition by Western blotting analysis | ||
| human LNCaP cells | Function assay | 10 μM | 2 h | Inhibition of autophosphorylation of immunoprecipitated flag-tagged Bmx expressed in human LNCaP cells assessed as incorporation of [32P]ATP at 10 uM pretreated for 2 hrs before transfection by immunoblot analysis | ||
| NCI-H1975 cells | Growth inhibition assay | 48 h | Inhibition of EGFR L858R/T790M mutant in human NCI-H1975 cells assessed as growth inhibition after 48 hrs by MTT assay | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
4-Methylpyridine : 100 mg/mL
DMSO
: Insoluble
Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 485.94 | Formula | C24H25ClFN5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 267243-28-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | PD183805 | Smiles | C=CC(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4 | ||
Mechanism of Action
| Features |
First kinase inhibitor to show irreversible activity and to have entered clinical trials (serving as a template for further development).
|
|---|---|
| Targets/IC50/Ki |
EGFR
(Cell-free assay) 1.5 nM
ErbB2
(Cell-free assay) 9.0 nM
|
| In vitro |
Canertinib (CI-1033) shows excellent potency for irreversible inhibition of erbB2 autophosphorylation in MDA-MB 453 cells, and also demonstrates high permeability in Caco-2 cells. This compound alone significantly suppresses constitutively activated Akt and MAP kinase, and in combination inhibits Akt while preventing increased levels of MAPK phosphorylation. It stimulates p27 expression and p38 phosphorylation in MDA-MB-453 cells. CI-1033 is highly specific to the erbB receptor family and not sensitive to PGFR, FGFR or IR even at 50 μM. It shows high levels of inhibition in A431 cells expressing EGFR with IC50 of 7.4 nM, and suppresses heregulin-stimulated tyrosine phosphorylation of erbB2, erbB3 and erbB4 with IC50 of 5, 14 and 10 nM, respectively. The compound also inhibits expression of pp62c-fos in response to heregulin. It is predicted to modify Cys773 covalently within the ATP binding site of the HER2 kinase and enhances destruction of both mature and immature ErbB-2 molecules. This compound induces a significant decrease in measurable phosphorylation of tyrosine residues 845 and 1068 of EGFR, which are responsible for Src and Ras/MAPK signaling respectively. The corresponding residues of Her-2, tyrosine residues 877 and 1248 are dephosphorylated significantly by it at a concentration of 3 μM or higher. CI could block EGFR internalization and increase the rate of apoptosis in primary osteosarcoma cells in a titratable fashion. In addition, it inhibits the proliferation of TT, TE2, TE6 and TE10 cells significantly at 0.1 nM. |
| Kinase Assay |
Tyrosine Kinase Assays
|
|
Enzyme assays for determination of IC50 are performed in 96-well filter plates in a total volume of 0.1 mL, containing 20 mM Hepes, pH 7.4, 50 mM sodium vanadate, 10 μM the ATP containing 0.5 mCi of [32P]ATP, 20 mg of polyglutamic acid/tyrosine, 10 ng of EGFR tyrosine kinase, and appropriate dilutions of Canertinib (CI-1033). All components except the ATP are added to the well and the plate is incubated with shaking for 10 min at 25 °C. The reaction is started by adding [32P]ATP, and the plate is incubated at 25 °C for another 10 min. The reaction is terminated by addition of 0.1 mL of 20% trichloroacetic acid (TCA). The plate is kept at 4 °C for at least 15 min to allow the substrate to precipitate. The wells are then washed five times with 0.2 mL of 10% TCA and 32P incorporation determined with a Wallac β plate counter.
|
|
| In vivo |
Canertinib (CI-1033) shows impressive activity against A431 xenografts in nude mice at 5 mg/kg of body weight. This compound (20 to 80 mg/kg/d) achieves a high degree of tumor regressions in H125 xenograft models. Its oral administration causes a marked inhibition of growth in TT, TE6 and TE10 xenografts in nude mice, without animal death and <10% weight loss. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pEGFR / EGFR / p-HER2 / HER2 / p-HER3 / HER3 / MUC4 p-FAK / FAK / p-AKT / AKT |
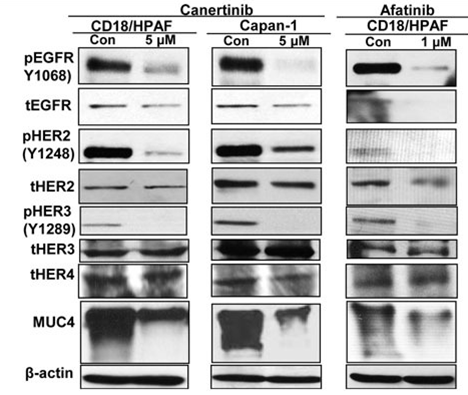
|
25686822 |
| Growth inhibition assay | Cell viability |
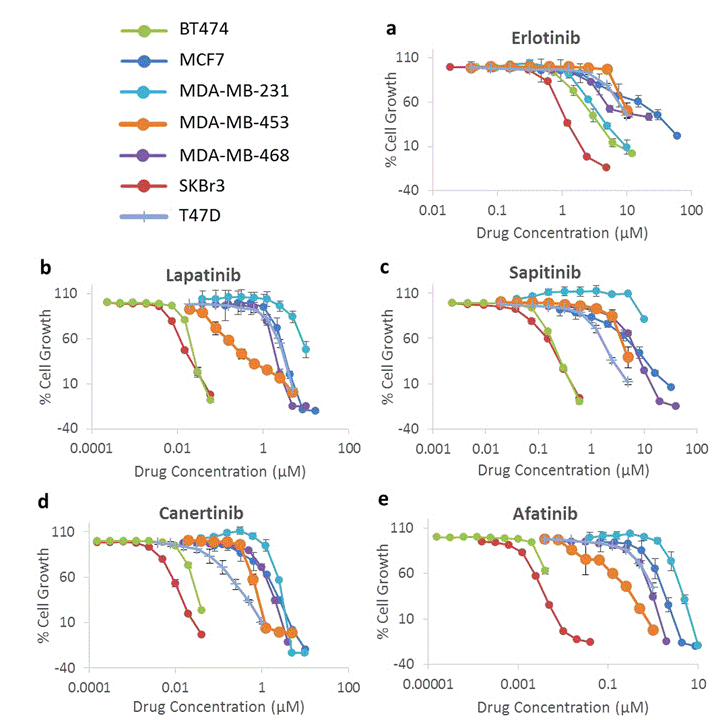
|
28638122 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00050830 | Completed | Lung Neoplasms |
Pfizer |
January 2003 | Phase 2 |
| NCT00174356 | Completed | Carcinoma Non-Small Cell Lung |
Pfizer |
December 2002 | Phase 1 |
| NCT00051051 | Completed | Breast Neoplasms |
Pfizer |
December 2002 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I would like to know which is the best option/solvent to dilute it for in vivo experiments. (I am treating mice at 30mg/mL of this compound.)
Answer:
It is a suspension in the formulation recommended (30% Propylene glycol, 5% Tween 80, 65% D5W) on our product page at 30mg/ml. It’s fine for oral gavage.






































