- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Nazartinib (EGF816) EGFR inhibitor
Cat.No.S7824
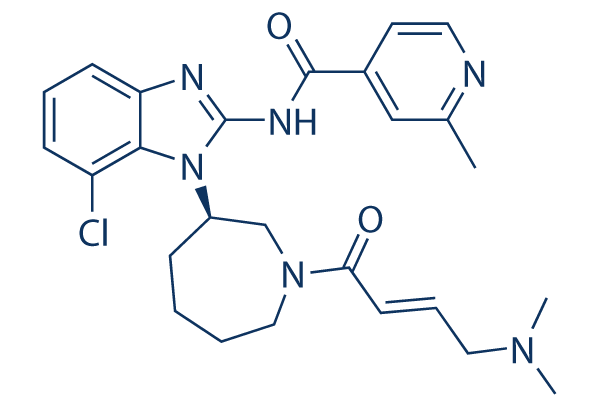
Chemical Structure
Molecular Weight: 495.02
Jump to
Quality Control
| Related Targets | VEGFR JAK PDGFR FGFR Src HIF FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other EGFR Products | Lazertinib (YH25448) Sunvozertinib Icotinib Hydrochloride AG-490 AG-1478 Canertinib (CI-1033) Rociletinib (CO-1686) WZ4002 Genistein Poziotinib (HM781-36B) |
Solubility
|
In vitro |
DMSO
: 99 mg/mL
(199.99 mM)
Ethanol : 99 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 495.02 | Formula | C26H31ClN6O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1508250-71-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NVS-816 | Smiles | CC1=NC=CC(=C1)C(=O)NC2=NC3=C(N2C4CCCCN(C4)C(=O)C=CCN(C)C)C(=CC=C3)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
mutant EGFR
0.031 μM(Ki)
|
|---|---|
| In vitro |
Nazartinib (EGF816) is a novel, covalent, mutant-selective EGFR inhibitor with nearly equipotent activity on both oncogenic (L858R and ex19del) and T790M-resistant mutations and good selectivity over WT EGFR. It potently inhibits the most common EGFR mutations L858R, Ex19del, and T790M in vitro. The cellular activity of this compound on EGFR mutants are assessed using three well-characterized cell lines, H3255, HCC827, and H1975, which harbor the L858R, Ex19del, and L858R/T790M mutations, respectively. After incubation with cells for 3 hours, it shows potent inhibition of pEGFR levels in H3255, HCC827, and H1975 with EC50 values of 5, 1, and 3 nmol/L, respectively. Cellular-based assays shows that EGF816 is selective toward mutant over WT EGFR.
|
| In vivo |
Nazartinib (EGF816) is well tolerated and possesses favorable physicochemical properties and good oral bioavailability in mice. It shows moderate volume of distribution and low to moderate clearance in rodents (30% and 35% of rat and mouse liver blood flow, respectively). In the dog, this compound shows high clearance and high volume of distribution. It also demonstrates antitumor activity in an exon 20 insertion mutant model. At levels above efficacious doses, its treatment leads to minimal inhibition of WT EGFR and is well tolerated. In single-dose studies, it provides sustained inhibition of EGFR phosphorylation, consistent with its ability for irreversible binding. This compound has a longer half-life in human than mouse and is currently being evaluated in phase I/II clinical trials in patients harboring EGFR mutations, including T790M.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03529084 | Withdrawn | Carcinoma Non-small Cell Lung |
Novartis Pharmaceuticals|Novartis |
July 24 2018 | Phase 3 |
| NCT03516214 | Completed | Bronchial Neoplasms |
University of Cologne |
April 25 2018 | Phase 1 |
| NCT03292133 | Active not recruiting | Lung Cancer |
Massachusetts General Hospital|Novartis |
October 31 2017 | Phase 2 |
| NCT02323126 | Terminated | Non Small Cell Lung Cancer |
Novartis Pharmaceuticals|Novartis |
February 9 2015 | Phase 2 |
| NCT02335944 | Terminated | Non Small Cell Lung Cancer |
Novartis Pharmaceuticals|Novartis |
January 13 2015 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































