Fluzoparib (SHR3162, HS10160) is a potent Poly (ADP-ribose) polymerase (PARP) inhibitor that shows anti-tumor activity, with an IC50 of 1.46±0.72 nM for PARP1.
- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Cat.No.S9712
| Related Targets | HDAC ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other PARP Products | XAV-939 AZD5305 (Saruparib) Veliparib (ABT-888) PJ34 HCl AG-14361 Iniparib (BSI-201) A-966492 G007-LK UPF 1069 AZD2461 |
|
In vitro |
DMSO
: 94 mg/mL
(198.98 mM)
Water : Insoluble Ethanol : Insoluble |
|
In vivo |
|||||
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
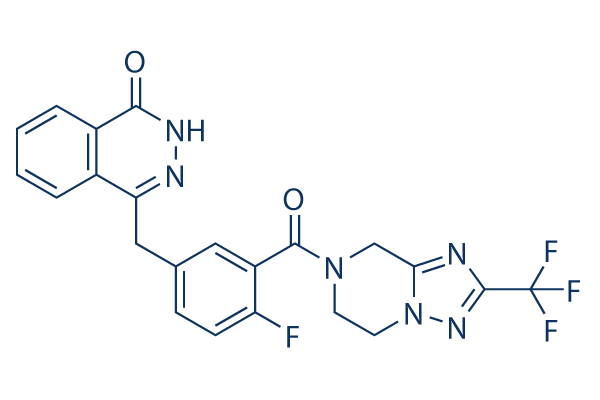
| Molecular Weight | 472.40 | Formula | C22H16F4N6O2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1358715-18-0 | -- | Storage of Stock Solutions |
|
|
| Synonyms | HS10160 | Smiles | FC1=CC=C(CC2=NNC(=O)C3=C2C=CC=C3)C=C1C(=O)N4CC[N]5N=C(N=C5C4)C(F)(F)F | ||
| Targets/IC50/Ki |
PARP
|
|---|---|
| In vitro |
Upon exposure to fluzoparib (SHR-3162), all four NSCLC cell lines (A549, H460, H1299, and PC9) show decreased viability in a time- and dose-dependent manner. It acts synergistically to inhibit cell growth and promote apoptosis among lung cancer cells. |
| In vivo |
An H460 cell xenograft mouse model is established to study the in vivo effects of fluzoparib (SHR-3162) and RT on tumor growth and survival. Treatment with RT + this compound more effectively delayed tumor growth than treatment with either RT or it individually. When combined with RT, it conferres a significant survival benefit with no evidence of toxicity. |
References |
|
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05732129 | Not yet recruiting | Homologous Recombination Deficiency Alterations Metastatic Colorectal Cancer |
Fudan University |
March 1 2023 | Phase 2 |
| NCT05032235 | Completed | Renal Impairment |
Jiangsu HengRui Medicine Co. Ltd. |
November 9 2021 | Phase 1 |
| NCT04694365 | Unknown status | Hepatic Impairment|Healthy Subjects |
Jiangsu HengRui Medicine Co. Ltd. |
November 6 2020 | Phase 1 |
| NCT04517357 | Unknown status | Relapsed Ovarian Cancer |
Jiangsu HengRui Medicine Co. Ltd. |
October 16 2020 | Phase 2 |
| NCT04659785 | Unknown status | Small Cell Lung Cancer Extensive Stage |
Tianjin Medical University Second Hospital |
July 1 2020 | Phase 1|Phase 2 |
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.