research use only
Pinometostat (EPZ5676) DOT1L inhibitor
Cat.No.S7062
Chemical Structure
Molecular Weight: 562.71
Quality Control
Products Often Used Together with Pinometostat (EPZ5676)
| Related Targets | HDAC JAK BET PKC PARP HIF PRMT EZH2 AMPK Histone Acetyltransferase |
|---|---|
| Other Histone Methyltransferase Inhibitors | 3-Deazaneplanocin A (DZNep) Hydrochloride BIX-01294 Trihydrochloride UNC1999 EPZ015666 (GSK3235025) EPZ004777 MM-102 (HMTase Inhibitor IX) Chaetocin SGC 0946 EPZ005687 UNC0638 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MV4-11 | Function assay | 4 days | Inhibition of DOT1L in human MV4-11 cells expressing MLL-AF4 assessed as reduction of H3K79me2 level after 4 days by ELISA method | 25406853 | ||
| MOLM13 | Proliferation assay | Antiproliferative activity against human MOLM13 cells containing MLL-AF9, EC50=4 nM | 23879463 | |||
| MV4-11 | Proliferation assay | Antiproliferative activity against human MV4-11 cells containing MLL-AF4, EC50=4 nM | 23879463 | |||
| THP1 | Proliferation assay | Antiproliferative activity against human THP1 cells containing MLL-AF9, EC50=4 nM | 23879463 | |||
| HeLa | Function assay | 72 hrs | Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISA, IC50 = 0.007 μM. | 28337327 | ||
| MV4-11 | Antiproliferative assay | 6 hrs | Antiproliferative activity against human MV4-11 cells harboring MLL-AF4 treated for 6 hrs measured after 8 days by Celltiter-Glo reagent based assay, IC50 = 0.015 μM. | 28337327 | ||
| MOLM13 | Function assay | 72 hrs | Inhibition of DOT1L in human MOLM13 cells assessed as suppression of HoxA9 gene after 72 hrs by luciferase reporter gene assay, IC50 = 0.052 μM. | 28337327 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(177.71 mM)
Ethanol : 25 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 562.71 | Formula | C30H42N8O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1380288-87-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C)N(CC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)O)C4CC(C4)CCC5=NC6=C(N5)C=C(C=C6)C(C)(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
DOT1L
(Cell-free assay) 80 pM(Ki)
|
|---|---|
| In vitro |
EPZ-5676 reduces H3K79 dimethylation with a cellular IC50 of 2.6 nM in MV4-11 cells. EPZ-5676 treatment results in concentration- and time-dependent reduction of H3K79 methylation without effect on the methylation status of other histone sites, which leads to inhibition of key MLL target genes and selective, apoptotic cell killing in MLL-rearranged leukemia cells. EPZ-5676 inhibits proliferation of MLL-AF4 rearranged cell line MV4-11 with an IC50 of 9 nM. |
| In vivo |
EPZ-5676 continuously intravenous infusion for 21 days to xenograft model of MLL-rearranged leukemia, leads to dose-dependent anti-tumor activity. At the highest dose of 70.5 mg/kg/day, complete tumor regressions are achieved with no regrowth for up to 32 days after the cessation of treatment. No significant weight loss or obvious toxicity is observed in rats treated with EPZ-5676 during efficacy study. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-p65 / p65 / NFATc1 CDK6 / BCL11A / Bcl-2 / RUNX1 / MEF2C / H3K79me3 / H4 |
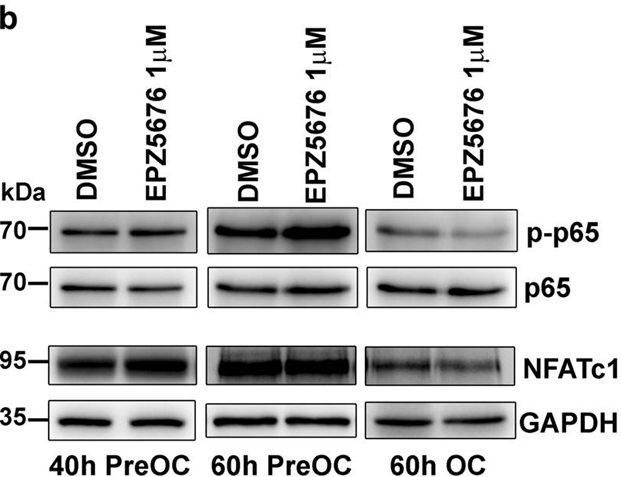
|
29348610 |
| Immunofluorescence | NFATc1 |
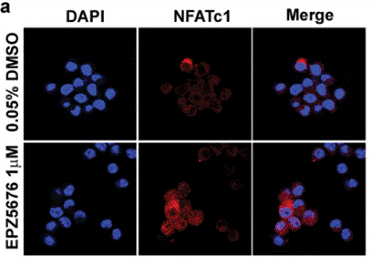
|
29348610 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02141828 | Completed | Leukemia|Acute Myeloid Leukemia|Acute Lymphocytic Leukemia|Acute Leukemias |
Epizyme Inc.|Celgene Corporation|Ipsen |
May 2014 | Phase 1 |
| NCT01684150 | Completed | Acute Myeloid Leukemia|Acute Lymphoblastic Leukemia|Myelodysplastic Syndrome|Myeloproliferative Disorders |
Epizyme Inc.|Celgene|Ipsen |
September 2012 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Is the vehicle 30% PEG400/0.5% Tween80/5% propylene glycol recommended for in vivo use?
Answer:
S7062 in 30% PEG400/0.5% Tween80/5% propylene glycol at 30 mg/ml is a suspension. For injection, 2% DMSO/30% PEG 300/5% Tween 80/ddH2O at 5 mg/ml is suitable.






































