research use only
UNC3866 Histone Methyltransferase antagonist
Cat.No.S8359
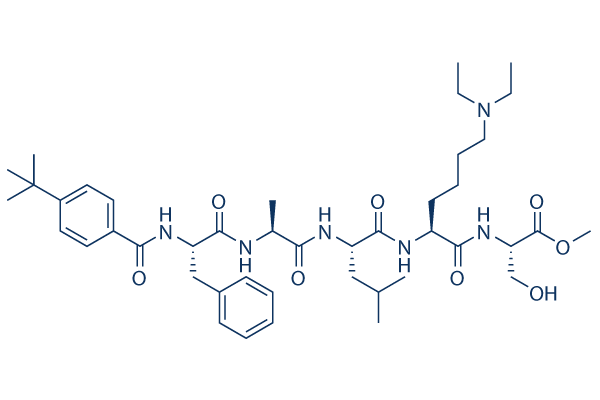
Chemical Structure
Molecular Weight: 795.02
Quality Control
| Related Targets | HDAC JAK BET PKC PARP HIF PRMT EZH2 AMPK Histone Acetyltransferase |
|---|---|
| Other Histone Methyltransferase Inhibitors | Pinometostat (EPZ5676) 3-Deazaneplanocin A (DZNep) Hydrochloride BIX-01294 trihydrochloride EPZ004777 EPZ015666 (GSK3235025) UNC1999 EPZ005687 SGC 0946 MM-102 UNC0638 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(125.78 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 795.02 | Formula | C43H66N6O8 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1872382-47-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCN(CC)CCCCC(C(=O)NC(CO)C(=O)OC)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C2=CC=C(C=C2)C(C)(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
PRC1 chromodomains
CBX4 chromodomains
94 nM(Kd)
CBX7 chromodomains
97 nM(Kd)
|
|---|---|
| In vitro |
UNC3866 is a potent antagonist of the CBX7-H3 interaction(IC50 = 66±1.2 nM). The affinity of this compound for CBX2, -4, -6 and -8 is also surprisingly well correlated with the percent sequence identity of each chromodomain relative to that of CBX7. It is equipotent for CBX4, which is most similar to CBX7, while it is 18-, 6- and 12-fold selective for CBX4/7 over CBX2, -6 and -8, respectively. Additionally, this chemical is 65-fold selective for CBX4/7 over CDY1 and 9-fold selective for CBX4/7 over CDYL1b and CDYL2. This compound antagonizes PRC1 chromodomains in cells. Thought the permeability of it is low, it is sufficiently cell permeable and stable to evaluate its ability to engage and antagonize the functions of its chromodomain targets in cells. It inhibits PC3 cell proliferation .
|
| In vivo |
UNC3866 is the predominant species in plasma at all time points tested relative to UNC4007 and shows 25% bioavailability and moderate clearance. While these PK results are promising for a peptidic compound, the use of this compound in vivo may be limited because of the high circulating levels required for intracellular target engagement due to its poor cell permeability. The potential utility of this chemical at higher doses for in vivo experiments is currently under investigation.
|
References |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































