research use only
Posaconazole P450 (e.g. CYP17) inhibitor
Cat.No.S1257
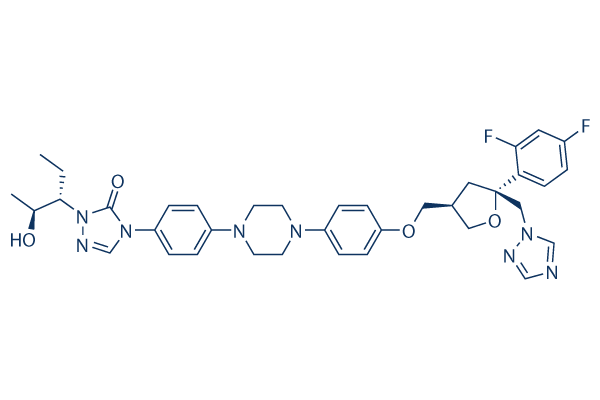
Chemical Structure
Molecular Weight: 700.78
Quality Control
| Related Targets | Dehydrogenase HSP Transferase PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other P450 (e.g. CYP17) Inhibitors | Apigenin Baicalein Avasimibe Naringenin Diosmetin Alizarin Sodium Danshensu Naringin Benzbromarone Orteronel |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human hepatocytes | Function assay | Inhibition of CYP3A4 in human hepatocytes using testosterone as substrate by HPLC/MS/MS method, IC50=0.05 μM | 24948565 | |||
| 3T3 | Antitrypanosomal assay | Antitrypanosomal activity against Trypanosoma cruzi Tulahuen infected in mouse 3T3 cells, EC50=0.0003μM | 19875282 | |||
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi epimastigotes infected in BESM cells assessed as accumulation of lanosterol and eburicol precursors at 5 uM after 72 hrs | 20385875 | |
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi epimastigotes infected in BESM cells assessed as reduction in episterol content at 5 uM after 72 hrs | 20385875 | |
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi epimastigotes infected in BESM cells assessed as reduction in fecosterol content at 5 uM after 72 hrs | 20385875 | |
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi amastigotes infected in BESM cells assessed as accumulation of lanosterol and eburicol precursors at 5 uM after 72 hrs | 20385875 | |
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi amastigotes infected in BESM cells assessed as reduction in episterol content at 5 uM after 72 hrs | 20385875 | |
| BESM | Function assay | 5 uM | 72 hrs | Inhibition of sterol 14-alpha-demethylase in Trypanosoma cruzi amastigotes infected in BESM cells assessed as reduction in fecosterol content at 5 uM after 72 hrs | 20385875 | |
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 20429511 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 20547819 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 20547819 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 20547819 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 23462713 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 24120539 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 24304150 | |||
| C2C12 | Function assay | 100 nM | 24 hrs | Inhibition of sterol biosynthesis in Trypanosoma cruzi CAI/72 amastigotes infected in mouse C2C12 cells assessed as increase in lanosterol level at 100 nM incubated for 24 hrs by GC-MS method | 25393646 | |
| C2C12 | Function assay | 100 nM | 24 hrs | Inhibition of sterol biosynthesis in Trypanosoma cruzi CAI/72 amastigotes infected in mouse C2C12 cells assessed as increase in eburicol level at 100 nM incubated for 24 hrs by GC-MS method | 25393646 | |
| C2C12 | Function assay | 100 nM | 24 hrs | Inhibition of sterol biosynthesis in Trypanosoma cruzi CAI/72 amastigotes infected in mouse C2C12 cells assessed as reduction in episterol level at 100 nM incubated for 24 hrs by GC-MS method | 25393646 | |
| C2C12 | Function assay | 100 nM | 24 hrs | Inhibition of sterol biosynthesis in Trypanosoma cruzi CAI/72 amastigotes infected in mouse C2C12 cells assessed as reduction in fecosterol level at 100 nM incubated for 24 hrs by GC-MS method | 25393646 | |
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 27014922 | |||
| ASZ | Function assay | 48 hrs | Inhibition of hedgehog pathway in mouse ASZ cells assessed as downregulation of Gli1 mRNA expression after 48 hrs by qPCR method, IC50=0.54μM | 27014922 | ||
| MERP MB | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse MERP MB cells assessed as cell growth inhibition using methyl-[3H]thymidine after 48 hrs by liquid scintillation spectrophotometry, GI50=1.5μM | 27014922 | ||
| J774 | Antileishmanial assay | 72 hrs | Antileishmanial activity against Leishmania amazonensis infected in mouse J774 cells after 72 hrs using 2 hrs parasite exposed mouse J774 cells, IC50=1.6μM | 27048943 | ||
| ASZ | Function assay | 48 hrs | Inhibition of hedgehog signaling pathway in mouse ASZ cells assessed as decrease in Gli1 mRNA expression after 48 hrs by qRT-PCR analysis, IC50=0.5μM | 30529635 | ||
| Ptch-CKO | Function assay | Inhibition of hedgehog signaling pathway in hedgehog-dependent mouse Ptch-CKO cells assessed as inhibition of cell growth, GI50=1.5μM | 30529635 | |||
| Caco2 | Function assay | Substrate activity at P-gp in human Caco2 cells by LC-MS/MS analysis | 30529635 | |||
| Vero | Antiviral assay | Antiviral activity against DENV2 16881 infected in African green monkey Vero cells by qRT-PCR analysis, EC50=4.1μM | 31128447 | |||
| Caco-2 | Function assay | 48 hrs | Determination of IC50 values for inhibition of SARS-CoV-2 induced cytotoxicity of Caco-2 cells after 48 hours by high content imaging, IC50=1.61μM | ChEMBL | ||
| Caco-2 | Function assay | 48 hrs | Toxicity against Caco-2 cells determined at 48 hours by intracellular ATP concentration using the CellTiter-Glo Luminescent Cell Viability Assay, CC50=14.64μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(142.69 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 700.78 | Formula | C37H42F2N8O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 171228-49-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | SCH 56592, POS | Smiles | CCC(C(C)O)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OCC5CC(OC5)(CN6C=NC=N6)C7=C(C=C(C=C7)F)F | ||
Mechanism of Action
| Features |
Currently the most advanced candidate for the treatment of Chagas disease.
|
|---|---|
| Targets/IC50/Ki |
lanosterol 14α-demethylase
CYP3A4
|
| In vitro |
Posaconazole has potent trypanocidal activity. Amiodarone acts synergistically with this compound. It also affects and disrupts Ca2+ homeostasis in T. cruzi. This chemical blocks the biosynthesis of ergosterol, which is essential for parasite survival. It has a clear, dose-dependent effect on proliferation of the epimastigote (extracellular) stages, with a minimal inhibitory concentration of 20 nM and an IC50 of 14 nM. Against the clinically relevant intracellular amastigote form of the parasite, this compound is even more potent. It has the minimal inhibitory concentration and IC50 values of 3 nM and 0.25 nM. It is active against isolates of Candida and Aspergillus spp. that exhibit resistance to Fluconazole, Voriconazole, and Amphotericin B and is much more active than the other triazoles against zygomycetes. |
| In vivo |
Treatment of infected animals with amiodarone alone reduces parasitemia, increases survival 60 days pi (0% for untreated controls vs 40% for amiodarone-treated animals) and, when given in combination with Posaconazole, delays the development of parasitemia. Coadministration of this compound and Boost Plus increases drug exposure compared to the administration of this compound alone in the fasted state. Food, particularly meals high in fat content, significantly increases this compound's bioavailability. Systemic exposure to this compound increases 4- and 2.6-fold when it is consumed with a high-fat and nonfat meal, respectively. This compound and Amiodarone may constitute an effective anti-T. cruzi therapy with low side effect. At twice-daily doses of ≥15 mg/kg of body weight, this compound prolongs the survival of the mice and reduces tissue burden. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
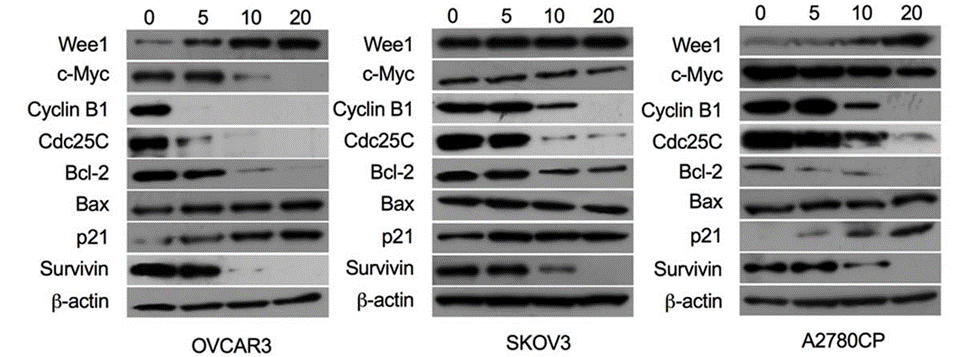
| Western blot | Wee1 / c-Myc / Cyclin B1 / Cdc25C / Bcl-2 / Bax / p21 / Survivin |
|
28383032 |
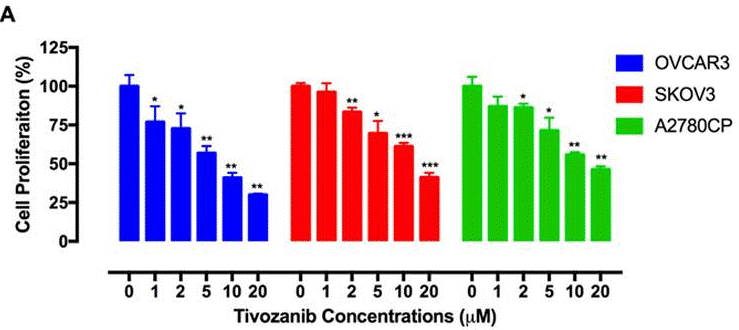
| Growth inhibition assay | Cell proliferation |
|
28383032 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06282718 | Not yet recruiting | Acute Respiratory Tract Infection |
European Clinical Research Alliance for Infectious Diseases (ECRAID)|UMC Utrecht|University of Oxford|Universiteit Antwerpen |
February 2024 | -- |
| NCT06158360 | Recruiting | Thyroid Cancer |
Ilsan Cha hospital |
January 1 2024 | -- |
| NCT05845359 | Withdrawn | Bariatric Surgery Candidate |
Montefiore Medical Center |
September 2023 | Phase 4 |
| NCT06302842 | Active not recruiting | Supportive Care |
Istituto Auxologico Italiano |
July 1 2023 | Not Applicable |
| NCT05617638 | Recruiting | Pain |
Hospital Israelita Albert Einstein|Beneficência Portuguesa de São Paulo |
June 27 2023 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































