- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Triptolide Hsp90 Middle Domain Inhibitor
Cat.No.S3604
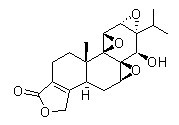
Chemical Structure
Molecular Weight: 360.4
Jump to
Quality Control
Batch:
Purity:
99.95%
99.95
| Related Targets | NF-κB HDAC Antioxidant ROS IκB/IKK Nrf2 AP-1 MALT NOD |
|---|---|
| Other ADC Cytotoxin Inhibitors | SN-38 (+)-Bicuculline Rutin Artemisinin Pinocembrin BHQ Harmine hydrochloride Psoralen Lappaconite HBr Luteoloside |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HT-29 | Cytotoxicity against | Cytotoxicity against human HT-29 cells, IC50=0.0021μM | 21470864 | |||
| HCT116 | Cytotoxicity against | Cytotoxicity against human HCT116 cells assessed as decrease in cell viability, IC50=0.0047μM | 31121546 | |||
| SKOV3 | Antiproliferative activity against | 72 hrs | Antiproliferative activity against human SKOV3 cells after 72 hrs by SRB assay, IC50=0.006μM | 20833543 | ||
| SKOV3 | Cytotoxicity against | 72 hrs | Cytotoxicity against human SKOV3 cells after 72 hrs by sulforhodamine B assay, IC50=0.006μM | 24378709 | ||
| SKOV3 | Cytotoxic activity against | 72 hrs | Cytotoxic activity against human SKOV3 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50=0.0072μM | 28011223 | ||
| KBM5 | Cytotoxicity against | 72 hrs | Cytotoxicity against imatinib-resistant human KBM5 cells harboring Bcr-Abl T315I mutant after 72 hrs by MTS assay, IC50=0.0083μM | 20149665 | ||
| SKOV3 | Cytotoxicity against | Cytotoxicity against human SKOV3 cells by SRB assay, IC50=0.009μM | 19637874 | |||
| MDA-MB-468 | Cytotoxicity against | Cytotoxicity against human MDA-MB-468 cells by SRB assay, IC50=0.01μM | 19637874 | |||
| HCT116 | Cytotoxicity against | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50=0.01μM | 19637874 | ||
| SKOV3 | Cytotoxicity against | 72 hrs | Cytotoxicity against human SKOV3 cells after 72 hrs by MTT assay, IC50=0.01μM | 19637874 | ||
| KBM5 | Cytotoxicity against | 72 hrs | Cytotoxicity against human KBM5 cells harboring wild type Bcr-Abl after 72 hrs by MTS assay, IC50=0.0103μM | 20149665 | ||
| Rh30 | Cytotoxicity against | 72 hrs | Cytotoxicity against human Rh30 cells after 72 hrs by MTT assay, IC50=0.014μM | 19637874 | ||
| A549 | Antagonist activity at | Antagonist activity at human PAR2 expressed in human A549 cells assessed as inhibition of 2f-LIGRLO-NH2-induced NFkappaB activation by luciferase reporter gene assay, IC50=0.014μM | 23895492 | |||
| SGC7901 | Cytotoxicity against | 72 hrs | Cytotoxicity against human SGC7901 cells after 72 hrs by MTT assay, IC50=0.015μM | 19637874 | ||
| MOLT4 | Cytotoxicity against | 72 hrs | Cytotoxicity against human MOLT4 cells after 72 hrs by MTT assay, IC50=0.017μM | 19637874 | ||
| A549 | Cytotoxic activity against | 72 hrs | Cytotoxic activity against human A549 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50=0.0175μM | 28011223 | ||
| SMMC7721 | Cytotoxicity against | 72 hrs | Cytotoxicity against human SMMC7721 cells after 72 hrs by MTT assay, IC50=0.018μM | 19637874 | ||
| PC3 | Cytotoxic activity against | 72 hrs | Cytotoxic activity against human PC3 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50=0.0183μM | 28011223 | ||
| MCF7 | Cytotoxicity against | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50=0.019μM | 19637874 | ||
| A549 | Cytotoxicity against | Cytotoxicity against human A549 cells, IC50=0.019μM | 21470864 | |||
| PC3 | Cytotoxicity against | Cytotoxicity against human PC3 cells by SRB assay, IC50=0.02μM | 19637874 | |||
| Bel7402 | Cytotoxicity against | 72 hrs | Cytotoxicity against human Bel7402 cells after 72 hrs by MTT assay, IC50=0.02μM | 19637874 | ||
| PC3 | Antiproliferative activity against | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by SRB assay, IC50=0.02μM | 20833543 | ||
| PC3 | Cytotoxicity against | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by sulforhodamine B assay, IC50=0.02μM | 24378709 | ||
| PC3 | Cytotoxicity against | Cytotoxicity against human PC3 cells assessed as inhibition of cell proliferation by sulforhodamine B assay, IC50=0.02μM | 25467158 | |||
| 786-O | Cytotoxicity against | 72 hrs | Cytotoxicity against human 786-O cells after 72 hrs by MTT assay, IC50=0.022μM | 19637874 | ||
| A549 | Antagonist activity at | Antagonist activity at human PAR2 expressed in human A549 cells coexpressing TACR1 assessed as inhibition of substance P-induced IL-8 production by ELISA, IC50=0.023μM | 23895492 | |||
| MDA-MB-231 | Cytotoxicity against | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50=0.024μM | 19637874 | ||
| DU145 | Cytotoxicity against | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by MTT assay, IC50=0.024μM | 19637874 | ||
| HO8910 | Cytotoxicity against | 72 hrs | Cytotoxicity against human HO8910 cells after 72 hrs by MTT assay, IC50=0.028μM | 19637874 | ||
| HCT15 | Cytotoxicity against | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by MTT assay, IC50=0.029μM | 19637874 | ||
| A549 | Growth inhibition of human | Growth inhibition of human A549 cells, IC50=0.03μM | 28814374 | |||
| 32D | Cytotoxicity against | 72 hrs | Cytotoxicity against mouse 32D cells harboring wild type Bcr-Abl after 72 hrs by MTS assay, IC50=0.032μM | 20149665 | ||
| U251 | Cytotoxicity against | Cytotoxicity against human U251 cells assessed as inhibition of cell proliferation by sulforhodamine B assay, IC50=0.033μM | 25467158 | |||
| 32D | Cytotoxicity against | 72 hrs | Cytotoxicity against imatinib-resistant mouse 32D cells harboring Bcr-Abl T315I mutant after 72 hrs by MTS assay, IC50=0.034μM | 20149665 | ||
| PC3 | Cytotoxicity against | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, IC50=0.043μM | 19637874 | ||
| KB | Cytotoxicity against | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by MTT assay, IC50=0.043μM | 19637874 | ||
| HepG2 | Cytotoxicity against | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by XTT assay, IC50=0.0433μM | 30613335 | ||
| HeLa | Cytotoxicity against | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50=0.047μM | 19637874 | ||
| U251 | Cytotoxicity against | 72 hrs | Cytotoxicity against human U251 cells after 72 hrs by MTT assay, IC50=0.049μM | 19637874 | ||
| K562 | Cytotoxicity against | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, IC50=0.05μM | 19637874 | ||
| NIH/3T3 | Cytotoxicity against | Cytotoxicity against mouse NIH/3T3 cells assessed as decrease in cell viability, IC50=0.05μM | 31121546 | |||
| SW1116 | Cytotoxicity against | 72 hrs | Cytotoxicity against human SW1116 cells after 72 hrs by MTT assay, IC50=0.052μM | 19637874 | ||
| A549 | Cytotoxicity against | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50=0.059μM | 19637874 | ||
| HeLa | Cytotoxicity against | Cytotoxicity against human HeLa cells assessed as decrease in cell viability, IC50=0.087μM | 31121546 | |||
| Jurkat | Cytotoxicity against | Cytotoxicity against human Jurkat cells assessed as decrease in cell viability, IC50=0.14μM | 31121546 | |||
| MKN28 | Cytotoxicity against | 72 hrs | Cytotoxicity against human MKN28 cells after 72 hrs by MTT assay, IC50=0.2μM | 19637874 | ||
| MDCK | Cytotoxicity against | Cytotoxicity against MDCK cells assessed as decrease in cell viability, IC50=1.2μM | 31121546 | |||
| Pkd1-/- | Induction of | Induction of cell growth arrest in mouse Pkd1-/- cells in presence of calcium | 17360534 | |||
| Pkd1-/- | Increase in | 100 nM | 96 hrs | Increase in p21CIP/WAF expression in mouse Pkd1-/- cells at 100 nM after 96 hrs by Western blot analysis | 17360534 | |
| Pkd1+/- | Increase in | 100 nM | Increase in PC2 dependent calcium release in mouse Pkd1+/- cells at 100 nM | 17360534 | ||
| Pkd1-/- | Increase in | 100 nM | Increase in PC2 dependent calcium release in mouse Pkd1-/- cells at 100 nM | 17360534 | ||
| Pkd1-/- | Increase in | 50 uM | Increase in calcium release in mouse Pkd1-/- cells at 50 uM in presence of RyR antagonist dantrolene | 17360534 | ||
| Pkd2+/- | Growth inhibition of PC2 expressing mouse | 100 nM | 24 hrs | Growth inhibition of PC2 expressing mouse Pkd2+/- cells as cell death at 100 nM after 24 hrs | 17360534 | |
| KBM5 | Cytotoxicity against | 0.001 to 17 uM | 72 hrs | Cytotoxicity against human KBM5 cells harboring wild type Bcr-Abl at 0.001 to 17 uM after 72 hrs by MTS assay | 20149665 | |
| KBM5 | Cytotoxicity against | 0.001 to 17 uM | 72 hrs | Cytotoxicity against imatinib-resistant human KBM5 cells harboring Bcr-Abl T315I mutant at 0.001 to 17 uM after 72 hrs by MTS assay | 20149665 | |
| LNCAP | Antagonist activity at | 5 uM | 24 hrs | Antagonist activity at AR in human LNCAP cells assessed as suppression of DHT-induced receptor transcriptional activity at 5 uM after 24 hrs by dual luciferase reporter gene assay | 27994731 | |
| LNCAP | Antagonist activity at | 500 nM | 24 hrs | Antagonist activity at AR in human LNCAP cells assessed as suppression of DHT-induced receptor transcriptional activity at 500 nM after 24 hrs by dual luciferase reporter gene assay | 27994731 | |
| HepG2 | Antitumor activity against | 0.2 mg/kg | 15 days | Antitumor activity against human HepG2 cells xenografted in Balb/c nude mouse assessed as reduction in tumor growth at 0.2 mg/kg, ip administered once daily for 15 days | 30613335 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 72 mg/mL
(199.77 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 360.4 | Formula | C20H24O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 38748-32-2 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
HSF1
MDM2
|
|---|---|
| In vitro |
Triptolide is a diterpene triepoxide with potent immunosuppressive and antiinflammatory properties. This compound is shown to inhibit the expression of IL-2 in activated T cells at the level of purine-box/nuclear factor and NF-κB mediated transcription activation. It inhibits the proliferation and colony formation of tumor cells at extremely low concentrations (2–10 ng/mL). This chemical has an inhibitory activity on breast, stomach and leukemia cell line HL-60 cells. It induces apoptosis in tumor cells by blocking NF-κB activation and sensitizing tumor cells for TNF-&alpha induced programmed cell death.
|
| In vivo |
Triptolide synergizes with cyclosporin A in promoting graft survival in animal models and in suppression of graft versus host disease in allogeneic bone marrow transplants. In addition, it induces apoptosis in tumor cells and potentiates tumor necrosis factor (TNF-α) induction of apoptosis in part through the suppression of c-IAP2 and c-IAP1 induction. This compound treatment for 2–3 weeks inhibits the growth of xenografts formed by four different tumor cell lines (B16 melanoma, MDA-435 breast cancer, TSU bladder cancer, and MGC80-3 gastric carcinoma), indicating that TPL has a broad spectrum of activity against tumors that contain both wild-type and mutant forms of p53. In addition, it inhibits experimental metastasis of B16F10 cells to the lungs and spleens of mice. It has in vitro and in vivo activities against mouse models of polycystic kidney disease. LD50: Mice 0.83mg/kg (i.v.).
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | c-Jun MDM2 p-AKT / AKT / p-Foxo3a / Foxo3a / p53 p-PI3K / PI3K / p85 / p110 |
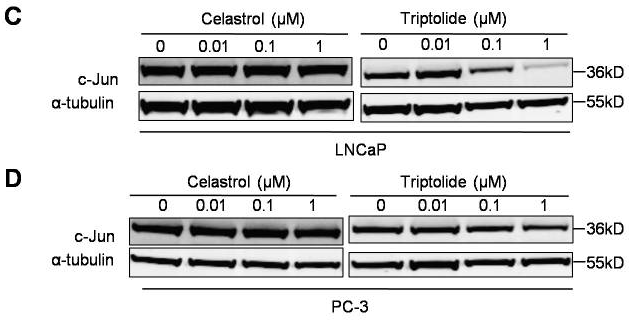
|
22666381 |
| Growth inhibition assay | Cell viability |
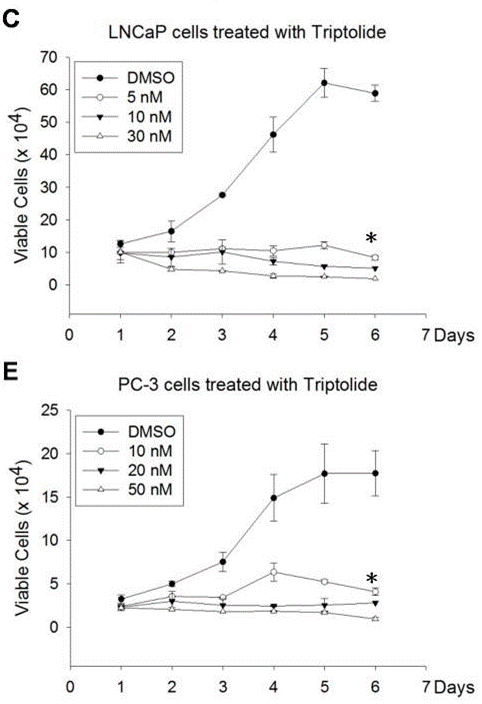
|
22666381 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































