research use only
Probenecid TRP Channel agonist
Cat.No.S4022
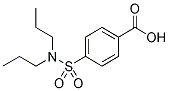
Chemical Structure
Molecular Weight: 285.36
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor ATPase GluR |
|---|---|
| Other TRP Channel Inhibitors | 2-APB (2-Aminoethyl Diphenylborinate) SKF96365 AMG-517 GSK2193874 GSK1016790A Capsazepine HC-030031 EIPA (L593754) HC-067047 SB705498 |
Solubility
|
In vitro |
DMSO
: 57 mg/mL
(199.74 mM)
Ethanol : 19 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 285.36 | Formula | C13H19NO4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 57-66-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Benemid | Smiles | CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
organic anion transport
TRPV2
TAS2R16
|
|---|---|
| In vitro |
Probenecid is able to prevent the efflux of calcium-sensitive fluorescent dyes during studies of cellular calcium mobilization. This compound (2.5 mM) is found to block the export of Fura-2 from 1321N1 astrocytoma cells, and does not change the basal calcium concentration or the muscarinic calcium response. It is a potent transient receptor potential vanilloid 2 (TRPV2) agonist. This chemical selectively induces increase in intracellular Ca2+ influx in HEK293T cells transiently expressing exogenous TRPV2. It is able to interact with organic anion transporters (OAT). 0.1 mM of this compound efficiently inhibits ATP-dependent active vesicular N-ethylmaleimide glutathione (NEM-GS) uptake by Human Multidrug Resistance Proteins 1 (MRP1) and MRP2. In isolated Sf9 cell membranes, it stimulates ATPase activity of MRP2 with approximate KACT of 250 μM, but inhibits ATPase activity of MRP1. This chemical is an inhibitor of the hTAS2R16, hTAS2R38, and hTAS2R43 bitter taste receptors. 1 mM of it attenuates saccharin-induced calcium flux responses of hTAS2R16 to near baseline levels. It dose-dependently inhibits hTAS2R16 (in the presence of 3 mM salicin) and hTAS2R38 (in the presence of 300 μM PTC). This activity is independent of its activity as a transport inhibitor, but is concerning with its interaction with the receptor. |
| In vivo |
Probenecid is an inhibitor of organic transport in vivo. This compound is able to decrease the renal clearance of antibiotic, such as penicillin, which enhances serum levels of antibiotic. It enhances the renal excretion of urate by acting as a competitive inhibitor of OAT, which prevents OAT-mediated reuptake of uric acid from the urine to the serum. This chemical is found to block acid metabolites from exiting the central nervous system, and is a competitive inhibitor of monoamine transport in the kidney, liver, and the eye. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04939623 | Recruiting | Chronic Pain|Drug Dependence of Morphine Type|Symptom Withdrawal |
University of Calgary |
October 31 2023 | Phase 2 |
| NCT05497674 | Completed | Type 2 Diabetes Mellitus |
Sunshine Lake Pharma Co. Ltd. |
February 21 2022 | Phase 1 |
| NCT05082909 | Unknown status | Infection Bacterial |
Imperial College London |
December 21 2021 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































