research use only
SB705498 TRP Channel antagonist
Cat.No.S2773
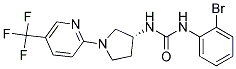
Chemical Structure
Molecular Weight: 429.23
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor ATPase GluR |
|---|---|
| Other TRP Channel Inhibitors | 2-APB (2-Aminoethyl Diphenylborinate) SKF96365 AMG-517 GSK2193874 GSK1016790A Capsazepine HC-030031 EIPA (L593754) HC-067047 ML-SI3 |
Solubility
|
In vitro |
DMSO
: 86 mg/mL
(200.35 mM)
Ethanol : 20 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 429.23 | Formula | C17H16BrF3N4O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 501951-42-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN(CC1NC(=O)NC2=CC=CC=C2Br)C3=NC=C(C=C3)C(F)(F)F | ||
Mechanism of Action
| Targets/IC50/Ki |
hTRPV1
7.6(pKi)
hTRPV1
7.1(pIC50)
|
|---|---|
| In vitro |
SB705498 (0.3 nM-1 μM) potently inhibits capsaicin-induced activation of human TRPV1 expressed in 1321N1 cells or HEK293 cells with apparent pKi of 7.5 or 7.6, respectively. Coapplication of 100 nM this compound rapidly, completely and reversibly inhibits hTRPV1 expressed in HEK293 cells. This chemical has no significant effect on endogenous [Ca2+] responses in HEK293 cells produced by muscarinic acetylcholine receptor activation with carbachol or store-operated channel-mediated Ca2+ entry after depletion of intracellular stores with the Ca2+ pump inhibitor thapsigargin. This compound (10 pM-1 μM) also has no significant antagonist effect versus the close TRPV1 receptor paralog TRPV4 transiently expressed in HEK293 cells and activated using the synthetic ligand 4α-phorbol-12,13-didecanoate (10 μM). This chemical reveals good antagonist potency against both the rat and guinea pig TRPV1. It antagonizes rat and guinea pig TRPV1 with pKi of 7.5 and 7.3, respectively. Coapplication of 100 nM to 10 μM this compound to the steady state of a maintained capsaicin response leads to rapid and complete inhibition of hTRPV1 at -70 mV. It inhibits capsaicin-mediated activation of hTRPV1 with IC50 of 3 nM and 17 nM at positive and negative holding potentials (-70 mV and + 70 mV), respectively. Coapplication of 1 μM this chemical to the plateau period of the response produces complete and reversible inhibition of the TRPV1-mediated conductance. This compound shows approximately equal activity versus multiple and diverse chemical and physical modes of TRPV1 receptor activation. It shows little or no activity versus a wide range of ion channels, receptors and enzymes. This chemical produces full blockade of heat as well as pH activation of hTRPV1.
|
| In vivo |
SB705498 exhibits potent and reversible blockade against the multiple modes of TRPV1 activation, namely the vanilloid (capsaicin), heat- and acid-mediated activation of the receptor. This compound displays excellent activity at 10 and 30 mg/kg po with good reversal of allodynia. It is also shown to give 80% reversal of allodynia in the guinea pig FCA model at 10 mg/kg p.o..
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01476098 | Completed | Rhinitis |
GlaxoSmithKline |
April 2011 | Phase 2 |
| NCT01424397 | Completed | Rhinitis |
GlaxoSmithKline |
April 14 2011 | Phase 2 |
| NCT01424514 | Completed | Rhinitis |
GlaxoSmithKline |
December 1 2010 | Phase 2 |
| NCT01439308 | Completed | Rhinitis |
GlaxoSmithKline |
December 2009 | Phase 2 |
| NCT00461682 | Terminated | Irritable Colon |
GlaxoSmithKline |
January 26 2007 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































