research use only
Vistusertib (AZD2014) mTOR inhibitor
Cat.No.S2783
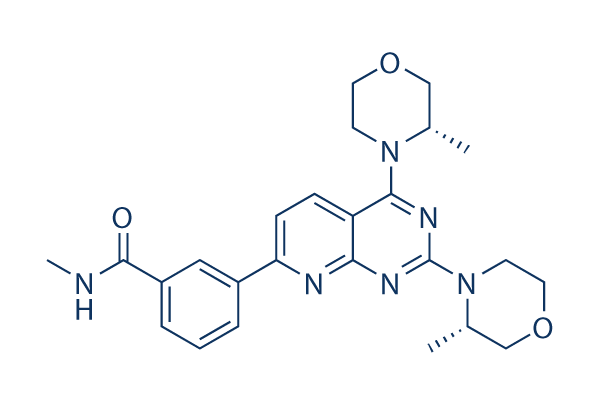
Chemical Structure
Molecular Weight: 462.54
Quality Control
| Related Targets | PI3K Akt GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other mTOR Inhibitors | Torin 1 Torin 2 AZD8055 Ridaforolimus (Deforolimus, MK-8669) Sapanisertib (MLN0128, INK-128) Torkinib (PP242) MHY1485 KU-0063794 OSI-027 WYE-354 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 | Function assay | Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells, IC50 = 0.0028 μM. | 23375793 | |||
| MDA-MB-468 | Function assay | 2 hrs | Inhibition of mTORC2 in human MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser473 after 2 hrs, IC50 = 0.08 μM. | 23375793 | ||
| MDA-MB-468 | Function assay | 2 hrs | Inhibition of mTORC1 in human MDA-MB-468 cells assessed as reduction of pS6 phosphorylation at Ser235/236 after 2 hrs, IC50 = 0.2 μM. | 23375793 | ||
| MCF7 | Function assay | Inhibition of mTORC2 in human MCF7 cells xenografted mouse assessed as modulation substrate | 23375793 | |||
| MCF7 | Function assay | Inhibition of mTORC1 in human MCF7 cells xenografted mouse assessed as modulation substrate | 23375793 | |||
| MCF7 | Cytotoxicity assay | 5 days | Cytotoxicity against human MCF7 cells after 5 days by Presto blue reagent-based fluorescence analysis | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 92 mg/mL
(198.9 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 462.54 | Formula | C25H30N6O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1009298-59-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1COCCN1C2=NC(=NC3=C2C=CC(=N3)C4=CC(=CC=C4)C(=O)NC)N5CCOCC5C | ||
Mechanism of Action
| Targets/IC50/Ki |
mTOR
(Cell-free assay) 2.8 nM
P-Akt (S473)
(Cell-free assay) 80 nM
pS6 (S235/236)
(Cell-free assay) 200 nM
|
|---|---|
| In vitro |
Vistusertib (AZD2014) is a close analogue of AZD8055 and a selective inhibitor of mTOR kinase. It has greater inhibitory activity against mTORC1 compared to rapamycin: this compound decreases p4EBP1 Thr37/46, inhibits the translation initiation complex and decreases overall protein synthesis while rapamycin has no effect. It also inhibits the mTORC2 biomarkers pAKTSer473 and pNDRG1Thr346. AZD2014 has broad antiproliferative activity across multiple tumour cell lines. In particular, it induces growth inhibition and cell death in breast cancer cell lines, including ER+ cell lines with acquired resistance to hormone therapy. |
| In vivo |
Vistusertib (AZD2014) induces tumour growth inhibition against several xenograft models including a human primary explant model of ER+ breast cancer. The antitumour activity is associated with modulation of both mTORC1 and mTORC2 substrates. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-4EBP1 / 4EBP1 / p-S6K / S6K / p-AKT S473 / AKT p-mTOR(S2448) / mTOR / p-S6(235/236) / S6 |
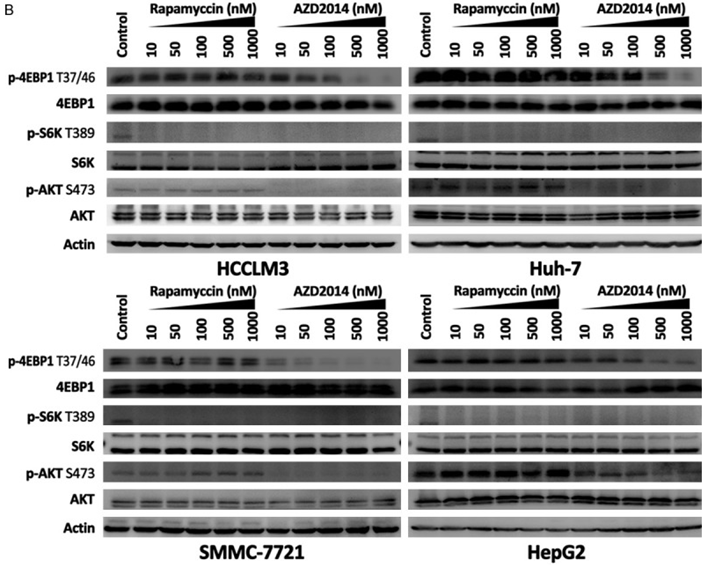
|
25628925 |
| Immunofluorescence | E-cadherin / Vimentin |
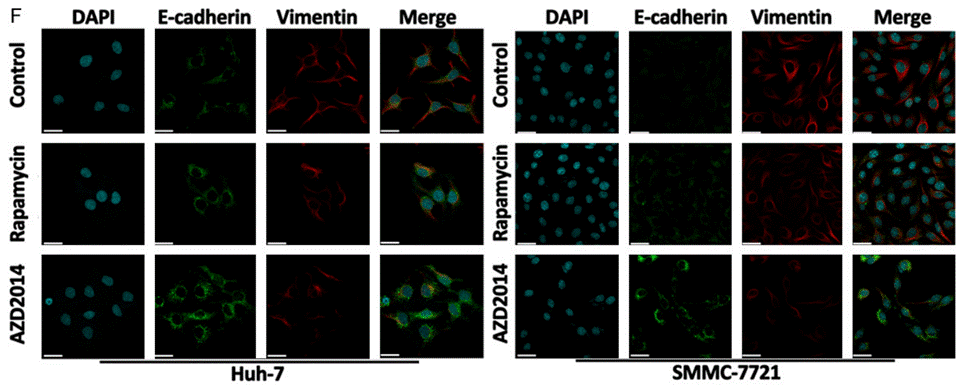
|
25628925 |
| Growth inhibition assay | Cell viability |
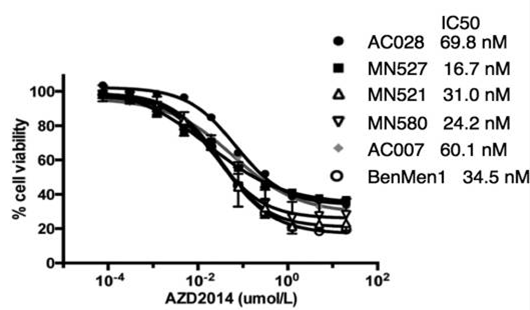
|
26219339 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02619864 | Completed | Glioblastoma Multiforme |
Canadian Cancer Trials Group|AstraZeneca |
December 22 2016 | Phase 1 |
| NCT02780830 | Withdrawn | Core: Relapsed or Refractory Diffuse Large B-Cell Lymphoma|Module 1: Non-GCB Diffuse Large B-Cell Lymphoma |
AstraZeneca |
June 2016 | Phase 1 |
| NCT02730923 | Active not recruiting | Endometrial Carcinoma|Metastatic Carcinoma|Hormone Receptor Positive Tumor |
Centre Leon Berard |
April 2016 | Phase 1|Phase 2 |
| NCT02599714 | Completed | Advanced and Metastatic Breast Cancer |
AstraZeneca |
December 7 2015 | Phase 1 |
| NCT02752204 | Completed | Diffuse Large B-Cell Lymphoma |
University of Birmingham|Bloodwise|AstraZeneca|Cancer Research UK |
October 2015 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I am looking for a i.p. or i.v. formula of it. Any suggestion?
Answer:
It can be dissolved in 5% DMSO/30% PEG 300/ddH2O at 5 mg/ml as a clear solution for I.P. use.






































