- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Zotarolimus (ABT-578) mTOR inhibitor
Cat.No.S7091
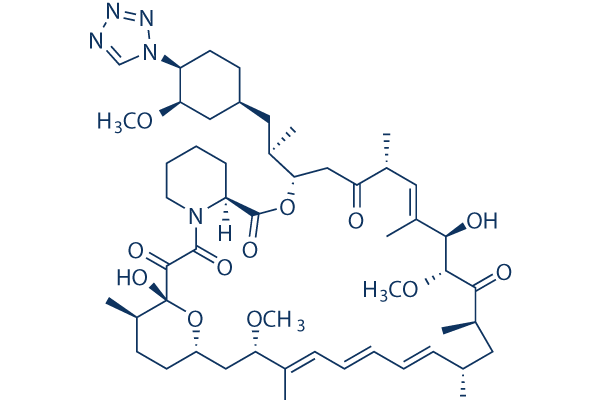
Chemical Structure
Molecular Weight: 966.21
Jump to
Quality Control
Batch:
Purity: >97%
97
| Related Targets | PI3K Akt GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other mTOR Products | Torin 1 Torin 2 AZD8055 Ridaforolimus (Deforolimus, MK-8669) Sapanisertib (INK-128) Torkinib (PP242) Vistusertib (AZD2014) MHY1485 KU-0063794 OSI-027 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(103.49 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 966.21 | Formula | C52H79N5O12 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 221877-54-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | A 179578 | Smiles | CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)N5C=NN=N5)C)C)O)OC)C)C)C)OC | ||
Mechanism of Action
| Features |
Zotarolimus has a shorter in vivo half-life and is also demonstrated in rats to have less potent systemic immunosuppression than rapamycin.
|
|---|---|
| Targets/IC50/Ki |
FKBP-12
2.8 nM
|
| In vitro |
Zotarolimus (ABT-578) is a semi-synthetic analogue of rapamycin, made by substituting a tetrazole ring for the native hydroxyl group at position 42 in rapamycin. This compound is highly effective in inhibiting both smooth muscle cell and endothelial cell proliferation, with IC50 values of 2.9 nM and 2.6 nM, respectively. It is mechanistically similar to sirolimus in having high-affinity binding to the immunophilin FKBP12 and comparable potency for inhibiting in vitro proliferation of both human and rat T cells. Zotarolimus inhibits Con A-induced human T cells and rat T cells proliferation with IC50 of 7.0 nM and 1337 nM respectively. |
| Kinase Assay |
Binding Affinity to FKBP12
|
|
96-well microtiter plates are first coated with FKBP-12 CMP-KDO synthetase fusion protein at 10 μg/mL, 100 μL/well for 2-3 h, followed by addition of 50 μL/well of buffer A (2% BSA and 0.2% Tween-20 in D-PBS) for 30-60 min. Microtiter plates are then washed three times with buffer B (0.2% Tween in D-PBS, pH adjusted to 7.4). Fifty microlitres of buffer A (for maximum), 20 μM FK506 in buffer A (for background), or various concentrations of zotarolimus (ABT-578) (10 pM-1 μM) in buffer A are added to each well followed by addition of 50 μL of A-79397 (an FK506 analogue)-alkaline phosphatase conjugate in buffer A. Microtiter plates are incubated at room temperature for 2-2.5 h followed by three washes with buffer B. About 100 μL of pNPP (p-nitrophenyl-phosphate) in 0.1 M aminomethylpropanol are added to each well and plates are incubated at room temperature for 90-120 min. Absorbance at 405 nM is read using an ELISA plate
|
|
| In vivo |
Zotarolimus (ABT-578) effectively reduces neointima formation in a 28-day, well-characterized swine model of coronary artery restenosis. It appears effective in preventing neointimal thickening, reducing late loss from 1.03 to 0.62 mm with a 47% reduction in TVF compared with bare metal stents (15.4% with the Driver stent to 8.1% with the Endeavor stent). This compound is efficacious in suppressing adjuvant DTH, EAE, and cardiac allograft rejection with ED50 values of 1.72, 1.17, and 3.71 mg/kg/day, respectively. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01239654 | Completed | Acute Coronary Syndrome |
IRCCS San Raffaele|Mediolanum Cardio Research|Cardiovascular Research Foundation New York |
September 2010 | Phase 3 |
| NCT01003717 | Completed | Coronary Artery Disease |
Rebecca Torguson|Medstar Health Research Institute |
October 2009 | -- |
| NCT00846846 | Completed | Coronary Artery Disease Autosomal Dominant 1 |
Medtronic Vascular |
January 2009 | Phase 4 |
| NCT00815139 | Completed | Coronary Artery Disease |
Yonsei University |
February 2008 | -- |
| NCT00599885 | Completed | Hypertension|Diabetes|Coronary Artery Disease |
Korea University Anam Hospital |
September 2007 | Phase 4 |
| NCT00476957 | Completed | Ischemic Heart Disease |
Medtronic Vascular|Medtronic Bakken Research Center |
June 2007 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































