research use only
Acelarin (NUC-1031) DNA/RNA Synthesis inhibitor
Cat.No.S9649
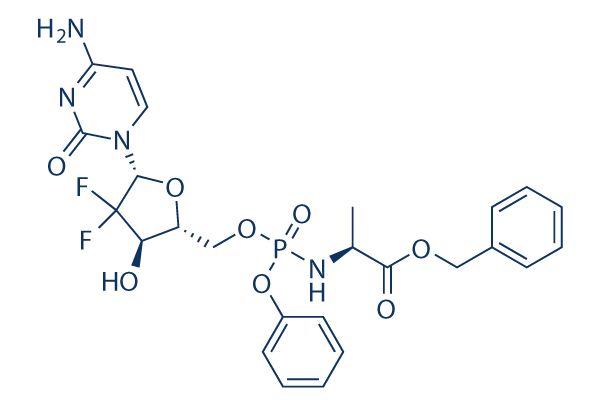
Chemical Structure
Molecular Weight: 580.47
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(172.27 mM)
Ethanol : 20 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 580.47 | Formula | C25H27F2N4O8P |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 840506-29-8 | -- | Storage of Stock Solutions |
|
|
| Synonyms | CPF-31, MTL-007, GTPL7389 | Smiles | CC(C(=O)OCC1=CC=CC=C1)NP(=O)(OCC2C(C(C(O2)N3C=CC(=NC3=O)N)(F)F)O)OC4=CC=CC=C4 | ||
Mechanism of Action
| Targets/IC50/Ki |
DNA synthesis
(Cell-free assay) 0.2 nM(EC50)
|
|---|---|
| In vitro |
Acelarin (NUC-1031) is a prodrug with potent cytostatic activity in a range of different tumor cell lines. Importantly, its activation is significantly less dependent on deoxycytidine kinase and on nucleoside transporters, and it is resistant to cytidine deaminase-mediated degradation. |
| In vivo |
In pancreatic cancer xenografts, Acelarin (NUC-1031) shows a significant reduction in tumor volumes in vivo. |
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02351765 | Completed | Biliary Tract Cancer|Gallbladder Cancer|Cholangiocarcinoma|Ampullary Cancer |
The Christie NHS Foundation Trust |
January 2016 | Phase 1 |
| NCT02303912 | Completed | Recurrent Ovarian Cancer |
Imperial College Healthcare NHS Trust |
November 2014 | Phase 1 |
| NCT01621854 | Completed | Cancer |
Imperial College London|Nucana |
October 2012 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































