research use only
SB415286 GSK-3 inhibitor
Cat.No.S2729
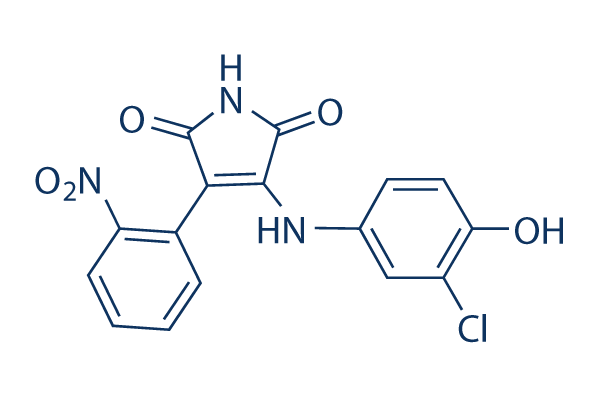
Chemical Structure
Molecular Weight: 359.72
Quality Control
| Related Targets | PI3K Akt mTOR ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other GSK-3 Inhibitors | CHIR-99021 (Laduviglusib) Laduviglusib (CHIR-99021) Hydrochloride SB216763 CHIR-98014 TWS119 GSK-3 Inhibitor IX (BIO) LY2090314 Tideglusib AR-A014418 IM-12 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CHO cells | Function assay | Stimulation of glycogen synthase in CHO cells expressing human insulin receptor, EC50=45.6 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 72 mg/mL
(200.15 mM)
Ethanol : 72 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 359.72 | Formula | C16H10ClN3O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 264218-23-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC=C(C(=C1)C2=C(C(=O)NC2=O)NC3=CC(=C(C=C3)O)Cl)[N+](=O)[O-] | ||
Mechanism of Action
| Targets/IC50/Ki |
GSK-3α
(Cell-free assay) 78 nM
GSK-3β
(Cell-free assay) ~78 nM
|
|---|---|
| In vitro |
SB 415286 inhibits GSK3α in an ATP competitive manner with Ki of 31 nM and shows similar potency against GSK3β. This compound has little or no activity against 24 other protein kinases with IC50 > 10 μ M. It stimulates glycogen synthesis in the Chang human liver cell line with EC50 of 2.9 μM, and induces expression of a β-catenin-LEF/TCF regulated reporter gene in HEK293 cells. It protects both central and peripheral nervous system neurones in culture from death induced by reduced PI3-kinase pathway activity in a concentration-dependent manner, which is correlated with inhibition of GSK-3 activity and modulation of GSK-3 substrates tau and β-catenin. In L6 myotubes, this chemical induces a much greater activation of GS (6.8-fold) compared to that elicited by insulin (4.2-fold) or Li (4-fold). This compound (10 μM) inhibits rapamycin-induced down-regulation of cyclin D1, and blocks rapamycin and -induced apoptosis, suggesting a critical role for GSK3β in rapamycin-mediated -sensitization. It prevents coxsackievirus-induced cell death in a dose-dependent manner via stabilization of β-catenin. This chemical exerts a protective effect on hydrogen peroxide-induced cell death in B65 rat neuroblastoma cells and neurons, while lithium does not attenuate the toxic effects of hydrogen peroxide. Its treatment potentiates TRAIL- and CH-11-induced apoptosis in HepG2 cells. Inhibition of GSK-3 by this compound causes multiple myeloma (MM) cell growth arrest and apoptosis through the activation of the intrinsic pathway. It decreases the viability of Neuro-2A cells, and induces the accumulation of cells in the G2/M phase of the cell cycle and subsequent apoptosis. |
| Kinase Assay |
GSK-3 activity assay
|
|
GSK-3 kinase activity is measured, in the presence of various concentrations of SB 415286, in a reaction mixture containing final concentrations of: 1 nM human GSK3α or rabbit GSK3α; 50 mM MOPS pH 7.0; 0.2 mM EDTA; 10 mM Mg-acetate; 7.5 mM L-mercaptoethanol; 5% (w/v) glycerol; 0.01% (w/v) Tween-20; 10% (v/v) DMSO; 28 μM GS-2 peptide substrate. The GS-2 peptide sequence corresponds to a region of glycogen synthase that is phosphorylated by GSK-3. The assay is initiated by the addition of 0.34 μCi [33P]γ-ATP (IC50 determinations) or 2.7 μCi [33P]γ-ATP (Ki determinations). The total ATP concentration is 10 μM (IC50 determinations) or ranged from 0 to 45 μM (Ki determinations). Following 30 minutes incubation at room temperature the assay is stopped by the addition of one third assay volume of 2.5% (v/v) H3PO4 containing 21 mM ATP. Samples are spotted onto P30 phosphocellulose mats and these are washed six times in 0.5% (v/v) H3PO4. The filter mats are sealed into sample bags containing Wallac betaplate scintillation fluid. 33P incorporation into the substrate peptide is determined by counting the mats in a Wallac microbeta scintillation counter.
|
|
| In vivo |
Administration of SB 415286 (~10 mg/kg twice daily) reduces the extent and degree of the trinitrobenzene sulphonic acid (TNBS)-provoked colonic inflammation in the rat, and reduces the fall in body weight, which is related to downregulation of NF-κB activity, involved in the generation of proinflammatory mediators. Treatment with this compound at 1 mg/kg significantly delays the growth of Neuro-2A cells in vivo in nude mice. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | β-catenin / XIAP / Bcl-2 GSK-3β / p53 |
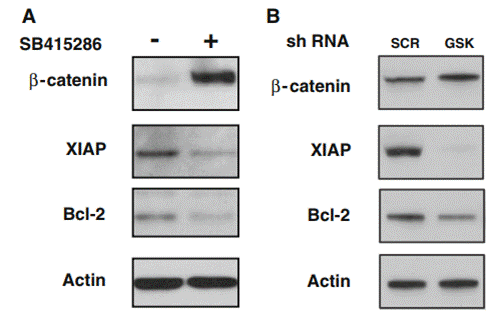
|
21161565 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































