research use only
Ropinirole HCl Dopamine Receptor inhibitor
Cat.No.S3189
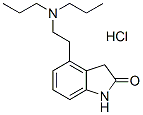
Chemical Structure
Molecular Weight: 296.84
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Dopamine Receptor Inhibitors | MPTP Hydrochloride Trifluoperazine Trifluoperazine 2HCl Penfluridol Sulpiride SCH-23390 hydrochloride Levosulpiride Domperidone SKF38393 HCl Rotundine |
Solubility
|
In vitro |
Water : 59 mg/mL
DMSO
: 30 mg/mL
(101.06 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 296.84 | Formula | C16H24N2O.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 91374-20-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | SKF-101468A | Smiles | CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
D2 receptor
29 nM(Ki)
|
|---|---|
| In vitro |
Ropinirole scavenges free radicals and suppresses lipid peroxidation in the Fe2+–H2O2 reaction system.
|
| In vivo |
Ropinirole (50 mg/kg, i.p.) causes biphasic spontaneous locomotor activity in mice. Ropinirole (0.05-1.0 mg/kg SC) dose-dependently inhibits the dyskinesias induced by 2-di-n-propylamino-5,6-di-hydroxytetralin in mice. Ropirtirole, at doses of 1 and 10 μg, injected unilaterally directly into the striatum of the rat causes marked, contralateral (away from the side of injection) asymmetry and circling in mice. Ropinirole (0.05-1.0 mg/kg SC or 0.1 mg/kg PO) reverses all motor and behavioural deficits induced by MPTP in marmosets. Ropinirole (2 mg/kg, i.p.) for 7 days increases GSH, catalase and SOD activities in the striatum and protected striatal dopaminergic neurons against 6-hydroxydopamine (6-OHDA) in mice. Ropinirole (0.2 mg/kg, i.p.) improves the use of previously akinetic forelimb and produced robust circling behavior in lesioned rats with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. The subtherapeutic dose of ropinirole generates only modest motor effects in lesioned rats with sole over-expression of D2R or D3R. Ropinirole (1-8 mg t.i.d.) is rapidly and completely absorbed with oral bioavailability of 55%, clearance of 780 mL/min, elimination half-life of 6 hours in healthy volunteer. Since the major route of elimination for Ropinirole is by the CYP enzyme system, mainly by CYP1A2 and also by CYP3A4, inhibition of the former and possibly the latter may reduce the agent’s clearance and lead to drug accumulation. Ropinirole (0.25 mg-4.0 mg per day) treatment significantly improves patients' ability to initiate sleep, the amount of stage 2 sleep and sleep adequacy compared with placebo. Periodic limb movements with arousal per hour decreases from 7.0 to 2.5 with ropinirole but increases from 4.2 to 6.0 with placebo. Periodic limb movements while awake per hour decreases from 56.5 to 23.6 with ropinirole but increases from 46.6 to 56.1 with placebo.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03708237 | Terminated | Restless Legs Syndrome|End Stage Renal Disease |
University of Alberta |
February 19 2019 | Phase 2 |
| NCT03250117 | Terminated | Parkinson Disease |
Titan Pharmaceuticals |
October 10 2017 | Phase 1|Phase 2 |
| NCT00823836 | Completed | Parkinson Disease |
GlaxoSmithKline |
March 2009 | Phase 3 |
| NCT00530790 | Completed | Restless Legs Syndrome |
GlaxoSmithKline |
August 23 2007 | Phase 2 |
| NCT00460148 | Completed | Parkinson Disease |
GlaxoSmithKline |
April 2007 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































