research use only
Palmitoylethanolamide PPAR activator
Cat.No.S4708
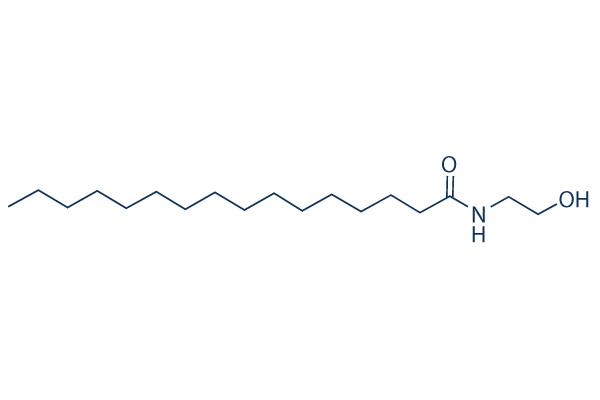
Chemical Structure
Molecular Weight: 299.49
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other PPAR Inhibitors | T0070907 GW9662 GW6471 WY-14643 (Pirinixic Acid) GSK3787 GW0742 AZ6102 Harmine Astaxanthin Eupatilin |
Solubility
|
In vitro |
Ethanol : 59 mg/mL
DMSO
: 5 mg/mL
(16.69 mM)
Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 299.49 | Formula | C18H37NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 544-31-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Palmidrol|N-palmitoylethanolamine | Smiles | CCCCCCCCCCCCCCCC(=O)NCCO | ||
Mechanism of Action
| Targets/IC50/Ki |
PPARα
(In HeLa cells) 3.1 μM(EC50)
|
|---|---|
| In vitro |
PEA protects cultured mouse cerebellar granule cells from glutamate toxicity and enhances microglial cell motility. In the mitochondrial fraction from cells stimulated with PEA, steroidogenic acute regulatory protein (StAR) and cytochrome P450 enzyme(P450scc) expression increases, both comprising proteins considered to be involved in crucial steps of neurosteroid formation. Moreover, PEA shows a protective effect, reducing malondialdehyde formation in cells treated with L-buthionine-(S,R)-sulfoximine, a glutathione depletor and the effect of PEA is partially inhibited by finasteride, a 5a-reductase inhibitor.
|
| In vivo |
PEA attenuates inflammation in wild-type mice but has no effect in mice deficient in PPAR-α. PEA has been shown to inhibit peripheral inflammation and mast-cell degranulation as well as to exert neuroprotective and antinociceptive effects in rats and mice. These actions are accompanied by changes in nitric oxide production, neutrophil influx, and expression of proinflammatory proteins such as inducible nitric oxide synthase and cyclooxygenase-2. In addition to its known anti-inflammatory activity, PEA regulates many pathophysiological
processes, including pain perception, convulsions, and neurotoxicity. In the central nervous system (CNS), where PEA is present at detectable levels, its concentrations significantly increase under
pathological conditions, such as excitotoxicity, brain ischaemia and neuroinflammation.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06040164 | Not yet recruiting | PreDiabetes|Diabetes Mellitus Type 2|Obesity|Oral Dysbiosis|Mouth Disease |
Fundación Pública Andaluza para la Investigación de Málaga en Biomedicina y Salud |
October 1 2023 | -- |
| NCT05849792 | Completed | Exercise|Physical Fitness|Depression|Adult ALL|Psychological |
University of Cadiz|Consejería de Salud y Familia (Junta de Andalucía)|Institute of Biomedical research and innovation of Cádiz (INIBICA) |
September 1 2019 | Not Applicable |
| NCT03564379 | Completed | Healthy |
Janssen Research & Development LLC |
June 12 2018 | Phase 1 |
| NCT01092845 | Completed | Healthy|Sleep |
Pfizer |
April 2010 | Phase 1 |
| NCT01370720 | Unknown status | Irritable Bowel Syndrome |
MARIA CRISTINA COMELLI|CM&D Pharma Limited |
February 2010 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































