research use only
Elafibranor PPAR agonist
Cat.No.S3720
Chemical Structure
Molecular Weight: 384.49
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other PPAR Inhibitors | T0070907 GW9662 GW6471 WY-14643 (Pirinixic Acid) GSK3787 GW0742 AZ6102 Harmine Astaxanthin Eupatilin |
Solubility
|
In vitro |
DMSO
: 76 mg/mL
(197.66 mM)
Ethanol : 60 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 384.49 | Formula | C22H24O4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 923978-27-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GFT505 | Smiles | CC1=CC(=CC(=C1OC(C)(C)C(=O)O)C)C=CC(=O)C2=CC=C(C=C2)SC | ||
Mechanism of Action
| Targets/IC50/Ki |
PPARα
PPARδ
|
|---|---|
| In vitro |
GFT505 is a novel PPAR modulator that shows a preferential activity on PPAR-α and concomitant activity on PPAR-δ. |
| In vivo |
Elafibranor (GFT505) is a dual PPARα/δ agonist that has demonstrated efficacy in disease models of nonalcoholic fatty liver disease (NAFLD)/NASH and liver fibrosis. In the rat, this compound concentrated in the liver with limited extrahepatic exposure and underwent extensive enterohepatic cycling. It confers liver protection by acting on several pathways involved in NASH pathogenesis, reducing steatosis, inflammation, and fibrosis. This chemical improved liver dysfunction markers, decreased hepatic lipid accumulation, and inhibited proinflammatory (interleukin-1 beta, tumor necrosis factor alpha, and F4/80) and profibrotic (transforming growth factor beta, tissue inhibitor of metalloproteinase 2, collagen type I, alpha 1, and collagen type I, alpha 2) gene expression. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p22phox / Nox-4 / LC3-II / Beclin-1 |
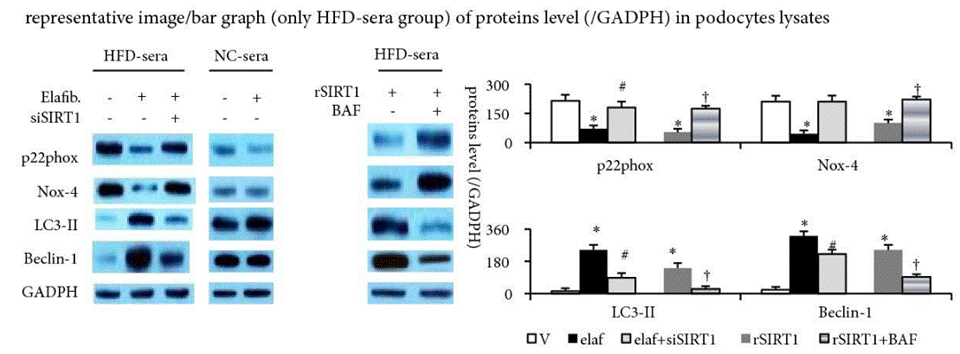
|
31321239 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05564208 | Completed | Healthy Participants |
Ipsen |
October 27 2022 | Phase 1 |
| NCT05543369 | Completed | Healthy Volunteers |
Ipsen |
September 19 2022 | Phase 1 |
| NCT04171752 | Completed | Geriatrics|Healthy |
Genfit |
November 22 2019 | Phase 1 |
| NCT03883607 | Terminated | Non Alcoholic Steatohepatitis |
Genfit |
June 25 2019 | Phase 2 |
| NCT03844555 | Completed | Renal Impairment|Renal Insufficiency|Kidney Diseases|Pharmacokinetics |
Genfit |
February 28 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































