research use only
Doxycycline MMP Inhibitor
Cat.No.S5159
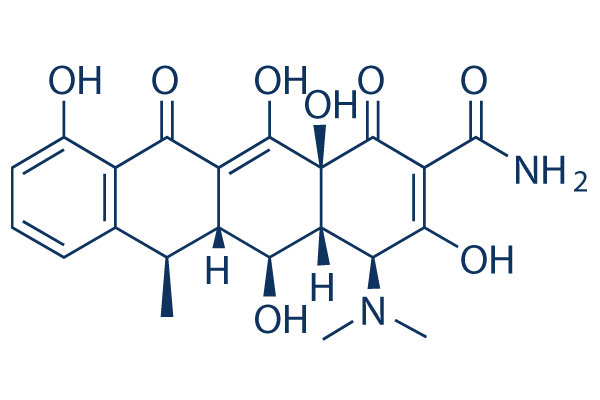
Chemical Structure
Molecular Weight: 444.43
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Other Antineoplastic and Immunosuppressive Antibiotics Inhibitors | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Staphylococcus aureus 2 planktonic cells | Bactericidal assay | 24 hrs | Bactericidal activity against methicillin-resistant Staphylococcus aureus 2 planktonic cells after 24 hrs by calgary biofilm device method, MBC=2μM. | 29638121 | ||
| THP1 | Function assay | Selectivity index, ratio of IC50 for human THP1 cells to IC50 for Plasmodium falciparum, IC50=3.1μM. | 19748781 | |||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19482476 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19926173 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19482476 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19926173 | ||
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells after 72 hrs by propidium iodide staining-based flow cytometry, IC50=20μM. | 19748781 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21741131 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 22889559 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21852132 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 24946216 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as cell viability after 72 hrs by MTT assay, CC50=20μM. | 25791675 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by MTT assay, CC50=20μM. | 25282267 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells measured after 72 hrs by MTT assay, CC50=20μM. | 27155463 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, CC50=20μM. | 27654395 | ||
| SW1353 | Function assay | 50 uM | Inhibition of IL-1-beta-induced MMP13 production in human SW1353 cells at 50 uM | 17267227 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against yafQ gene-deficient Escherichia coli K-12 BW25113 biofilm assessed as log reduction of viable cells after 24 hrs | 19307375 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against Escherichia coli K-12 BW25113 biofilm harboring pCA24N ptac::yafQ plasmid assessed as log reduction of viable cells after 24 hrs pretreated with 5 uM of IPTG for 4 hrs | 19307375 | ||
| Neuro2a | Function assay | 1 uM | Decrease in 7-DHC levels in Dhcr7-deficient mouse Neuro2a cells at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| vascular endothelial cells | Antibacterial assay | 25 ug/ml | 72 hrs | Antibacterial activity against Rickettsia prowazekii str. Breinl infected in CD rat primary pulmonary vascular endothelial cells assessed as bacterial shape change at 25 ug/ml measured 72 hrs post infection by Hoechst 33258/phalloidin staining based fluor | 28089350 | |
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Hep2 | Anti-Chlamydial assay | 1 ug/ml | 8 hrs | Anti-Chlamydial activity against Chlamydia trachomatis Serovar LGV-L2 infected in Hep2 cells assessed as reduction in size and number of chlamydial inclusion at 1 ug/ml treated at 8 hrs post-infection and 24 hrs later re-infecting fresh Hep2 cells monolay | 32227948 | |
| skeletal myoblast cells | Cytotoxicity assay | DNDI: Cytotoxicity in Vitro, 72 hour, in rat skeletal myoblast cells, IC50=14.49μM. | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(200.25 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 444.43 | Formula | C22H24N2O8 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 564-25-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Vibramycin, Doxytetracycline, Doxiciclina, Doxycyclinum | Smiles | CC1C2C(C3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | ||
Mechanism of Action
| In vitro |
100 ng/mL-5 µg/mL Doxycycline can significantly alter the metabolic profile of the cell, as well as reduce the proliferative rate, though the effect size depends upon the particular cell line used. This compound arrests cells in G1-S phase of the cell cycle in PANC-1 cells and induces the expression of p53 and its downstream target p21. It down-regulates antiapoptotic genes and induces proapoptotic genes. This chemical also induces apoptosis through a Fas/Fas-ligand dependent pathway in Jurkat T lymphocytes. |
|---|---|
| In vivo |
Doxycycline successfully reduces tumor growth in vivo (inhibited pancreatic cancer cell growth). |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
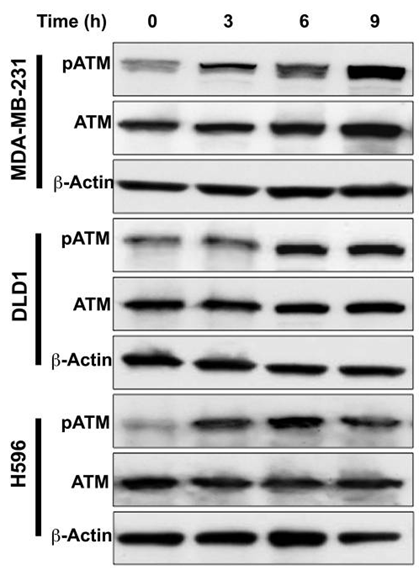
| Western blot | pATM / ATM HSP70 / HSP90 AKT / BCL6 / Cyclin E / HDAC2 / HDAC3 / NEMO / TYK2 / RIPK1 |
|
23282955 |
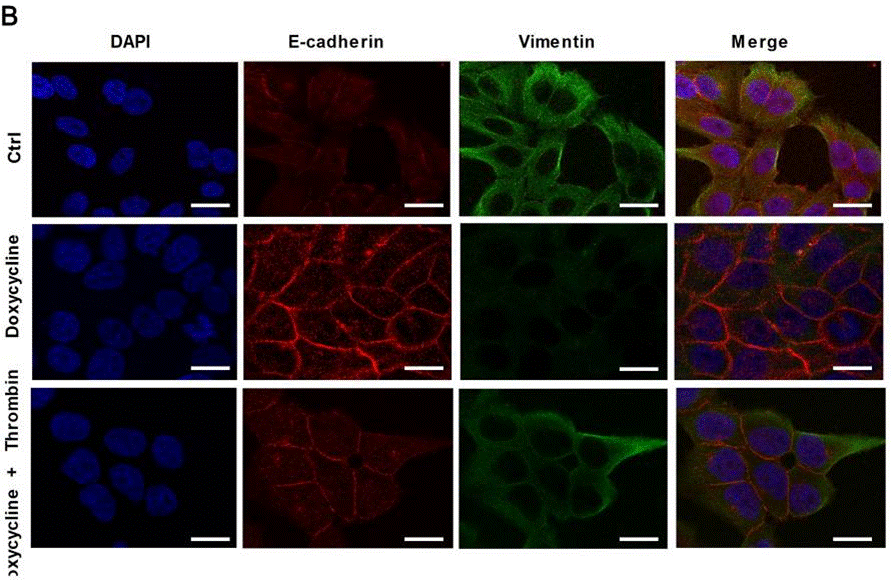
| Immunofluorescence | E-cadherin / Vimentin |
|
29285218 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05972772 | Not yet recruiting | Infectious Disease|Therapeutics |
Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit|Mahidol Oxford Tropical Medicine Research Unit |
March 20 2024 | Phase 2|Phase 3 |
| NCT06007534 | Recruiting | Post-exposure Prophylaxis|Sexually Transmitted Diseases|Doxycycline |
Assistance Publique - Hôpitaux de Paris |
October 25 2023 | Not Applicable |
| NCT04762134 | Recruiting | Bacterial Sexually Transmitted Diseases |
Jonathan Troy Grennan|Canadian Institutes of Health Research (CIHR)|British Columbia Centre for Disease Control |
June 2 2023 | Phase 4 |
| NCT05853120 | Recruiting | Sexually Transmitted Diseases |
Emory University|Centers for Disease Control and Prevention |
May 31 2023 | Phase 4 |
| NCT05382208 | Recruiting | Emphysema|HIV |
Weill Medical College of Cornell University|National Heart Lung and Blood Institute (NHLBI)|University of California Los Angeles|University of Iowa|University of Michigan |
August 22 2022 | Phase 2 |
| NCT05492019 | Recruiting | Parkinson Disease |
Bangabandhu Sheikh Mujib Medical University Dhaka Bangladesh |
July 1 2022 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































